The Lewis Dot Structure Of Nh3 Will Include A Double Bond
No lone pair is present on the central or outer atom in the lewis structure of ethene. In the Lewis Structure electrons are depicted as dots.

Dot And Cross Structure For Nh3 Ammonia Youtube
4 pts Draw the Lewis dot structure of NH3.

The lewis dot structure of nh3 will include a double bond. 4 pts Draw the Lewis dot structure of NH3. Also list the number of electron groups the electron pair geometry bond angle and the molecular geometry. The carbon is sp hybridized and oxygens are sp2 hybridized.
In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. The NH 3 Lewis structure has the typical case of nitrogen N in the center with 3 bonds to 3 other atoms. There is a double bond between each oxygen and carbon.
Count total valence electron in C2H4. What S The Lewis Dot Structure For Nh3 structure of H2O NH3 CH4 Becl2 Brainly in organic chemistry How to know when electrons will be What are the hybridizations of nitrogen atoms in Hydrazoic What is the vsepr model for NH3. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom.
How many valence electrons should you use to draw a Lewis structure of ammonia NH3. Osmoregulation in the cartilaginous fishes draw electron dot structure of co2 and NH3 Brainly in Which of the following has the smaller bond angle. If the rules for drawing Lewis electron dot diagrams do not work as written a double bond.
Normally however we draw N with a pair of dots on top. These structures provide information about the types of bonds single double or triple as well as the connectivity of atoms. NH3 Lewis structure ammonia electron dot structure is that type of diagram where we show the total eight valence electrons of NH3 as dots or dots and dashes-.
La représentation de Lewis dune molécule fait apparaître la position de tous les doublets électroniques liants et non liants autour des atomes qui la composent. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. A step-by-step explanation of how to draw the H3O Lewis Structure Hydronium Ion.
Draw the Lewis-Kekule structure of NH3 and N2. Hence the Lewis-dot structure of is shown below. This molecule is polar see above picture.
The bond between the C and O atoms is a double bond and represents two bonding pairs of electrons between the atoms. Lewis Dot Structure Nh3 Covalent Bond What is the Lewis structure of NH3. E Définition de Lewis.
The oxygens are sp3 hybridized. The H C Calculating NH3 Formal Charges. Lewis Structure is a simplified arrangement and presentation of the electrons present in the valence shell of a molecule.
In NH3all hydrogens follows the duet rule and the. NH3 H NH4 Lewis Structure. Formula for formal charge.
This unit looks more in depth at molecular compounds to see how they are bonded together by looking at Lewis structures. According to Lewis-dot structure there are 6 number of bonding electrons and 2 number of non-bonding electrons. HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.
If we were to use lines to represent the bonds we would use two lines between the C and O atoms. Lewis Dot Structure Of Nh3 Exam 3. There is also a lone pair of electrons unbonded electrons on N.
Each valence shell is full so this is an acceptable Lewis electron dot diagram. H2O- Bond angles are less than 1095 degrees 1039 as analyzed with chime on the class website which is expected from this molecules hybridization. Also list the number of electron groups the electron pair geometry bond angle and the molecular geometry.
A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. La définition de Bronsted-Lowry bien que résolvant une partie des problèmes qui se posaient avec la théorie d. The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1.
Now we have to determine the formal charge for each atom. Study Covalent Bonds NH4 Lewis Structure How to Draw the Dot Structure for NF3 Lewis Structure How to Draw the Dot Structure for. The lone pair dots on N could be drawn in any position up down left or right.
For the H3O Lewis structure we first count the valence electrons for. By knowing the Lewis structure we can also predict the three-dimensional geometry of an individual molecule.
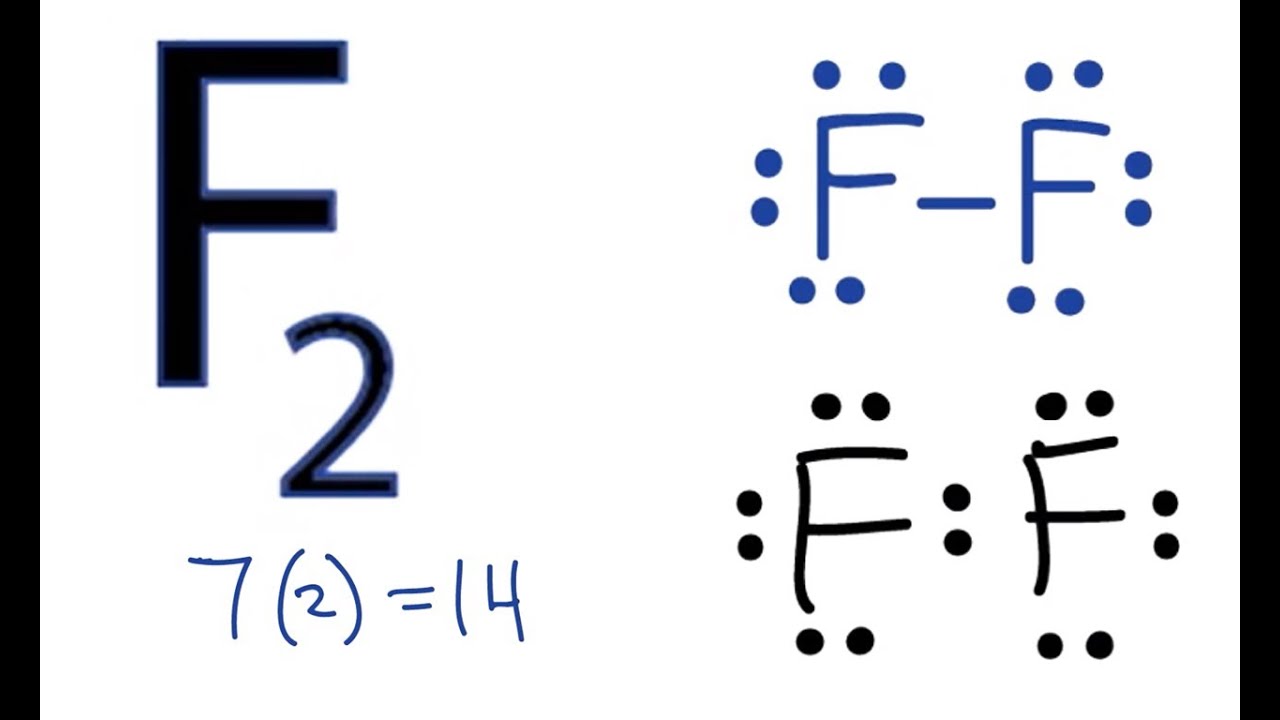
F2 Lewis Structure How To Draw The Lewis Dot Structure For F2 Youtube
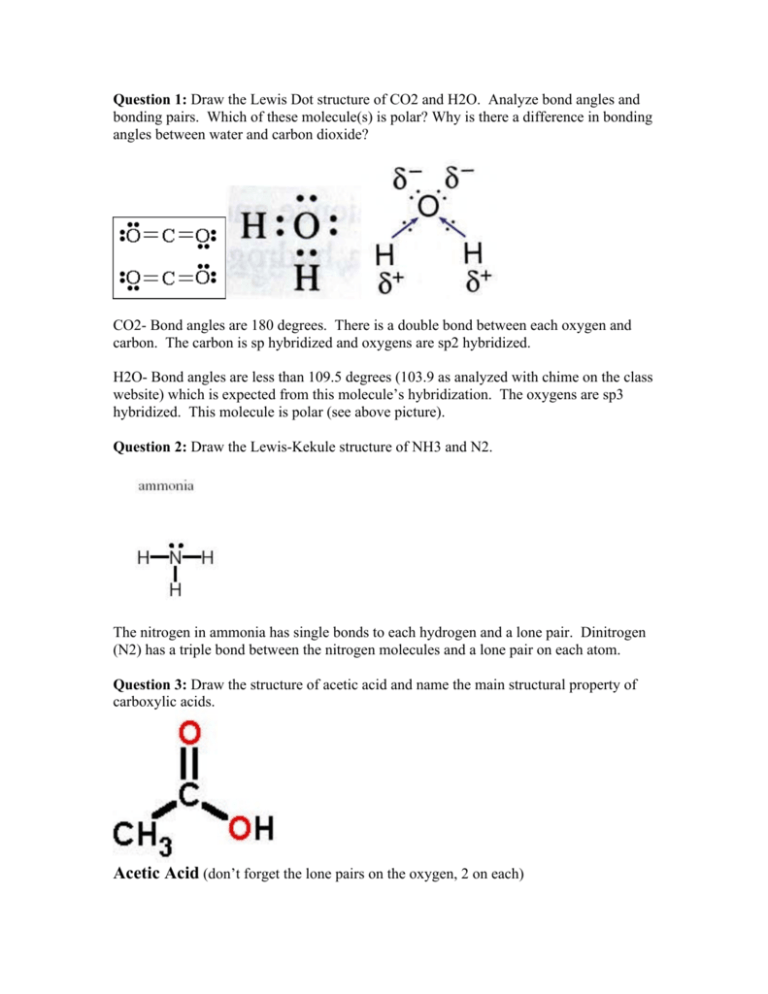
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

3 Ways To Draw Lewis Dot Structures Wikihow

How To Draw The Lewis Dot Structure For H3po3 Phosphorous Acid Youtube
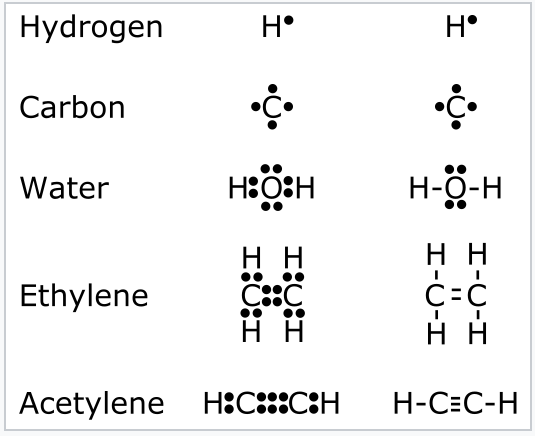
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Nh3 Lewis Structure Ammonia Youtube

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In

Draw The Electron Dot Structure Of Ammonia Molecule And Show The Formation Of Ammonium Ion From Meet Brainly In

What Does The Lewis Structure Of Na2so4 Look Like Study Com
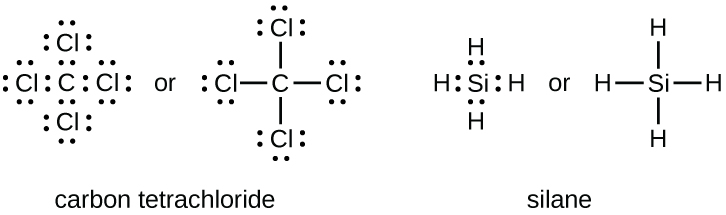
4 2 Lewis Structures Chemistry Libretexts

Lewis Dot Structure Practice Flashcards Quizlet

Lewis Dot Structure For Nitrogen Atom N Youtube

Lewis Structures 2 Water And Ammonia Youtube

Draw The Electron Dot Structure Of Ammonia Molecule And Show The Formation Of Ammonium Ion From Meet Brainly In

How To Draw The Lewis Dot Structure For Ch4 Methane Youtube

Lewis Dot Structure Practice Problems With Answers And Explanation Youtube

Trick To Draw Lewis Dot Structures Youtube