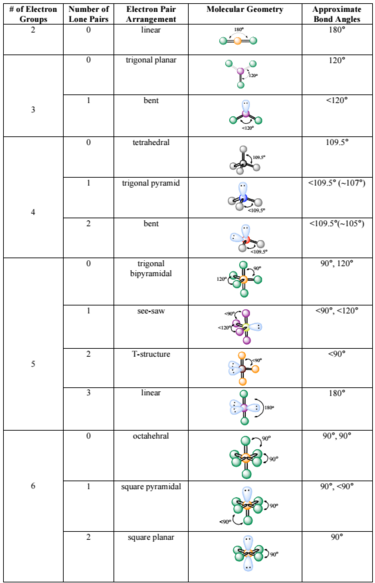
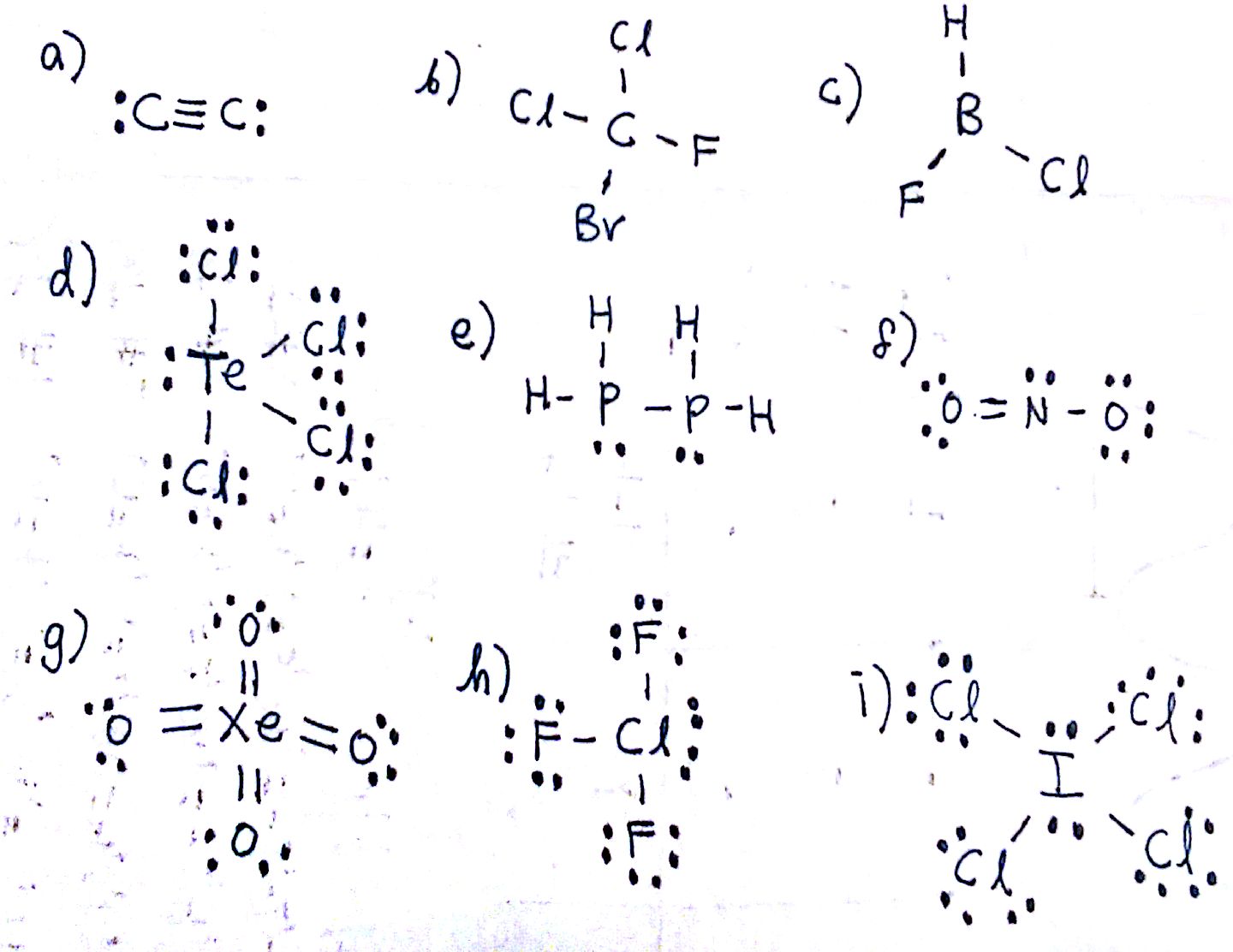C2h4 Lewis Structure Lone Pairs
To know the reason why c2h4 has two ch and four h molecules. How many electrons are shared between the carbon atoms in.

Localized And Delocalized Lone Pairs And Bonds Chemistry Steps
1 CC bond 2 shared pairs of electrons.
C2h4 lewis structure lone pairs. Dome7w and 13 more users found this answer helpful. Total number of shared pair of electrons 42 6. In CO Lewis structurewe get five pairs of electronsOut of five pairs of electronsCO has three bond pairs and two lone pairs of electronsHere carbon has one lone pair and oxygen has one lone pair of electrons.
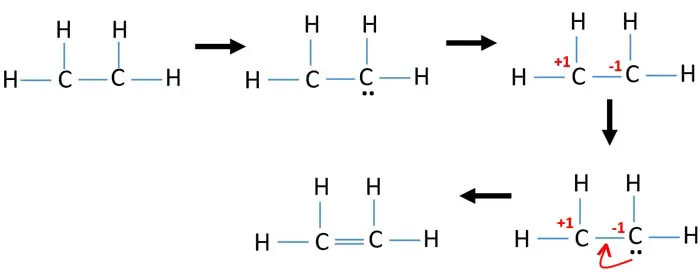
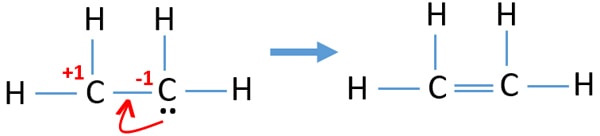
The CC bond is a covalent bond made up of a sigma bond and a pi bond where 4 electrons are shared two from each C. There are no lone pairs in this Lewis structure because all of the valence electrons are being shared in bonds. Now we are going to draw that C 2 H 4 lewis structure.
How many single bonds are there. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. Count total valence electron in C2H4.
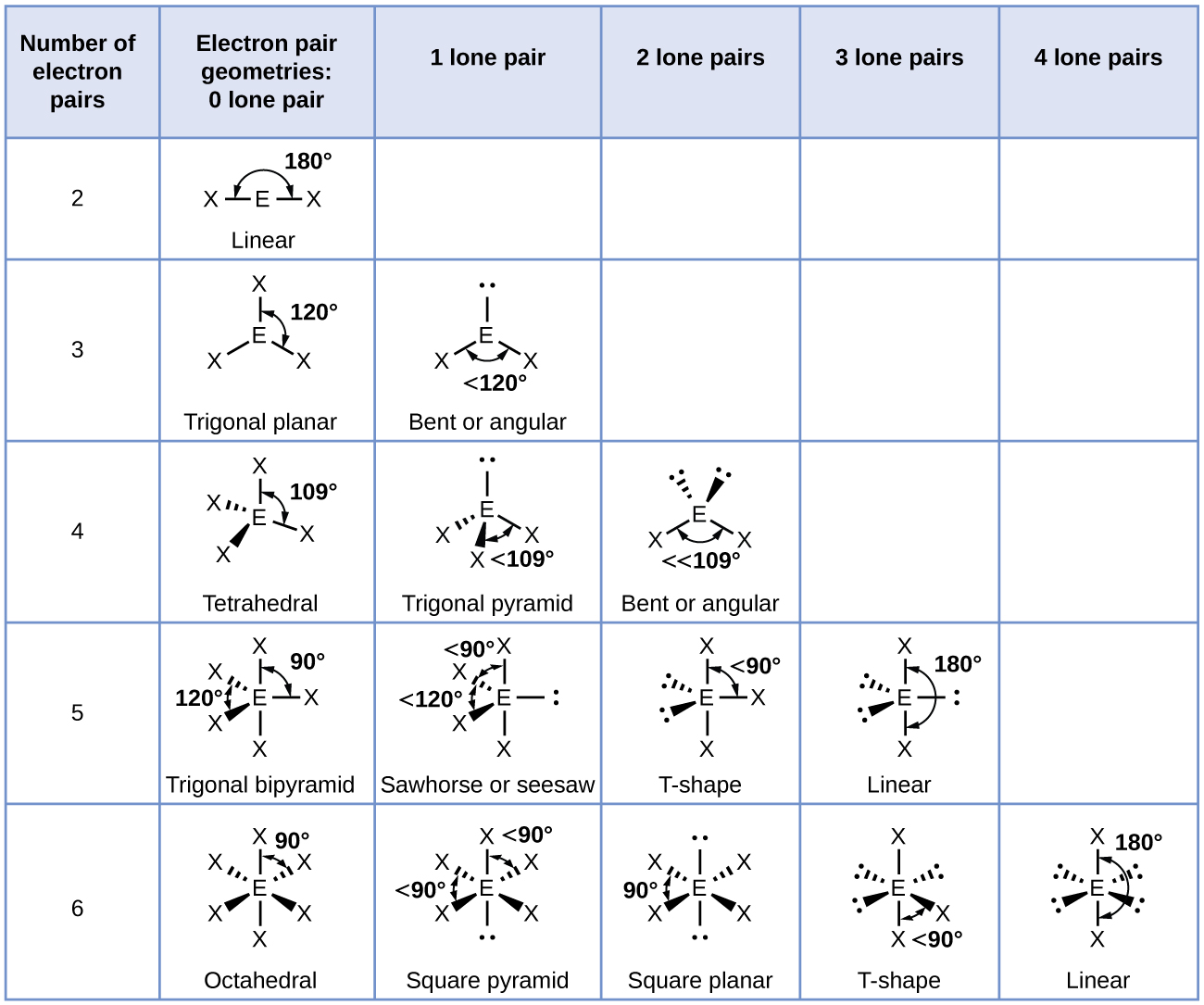
According to the VSEPR chart the shape of the ethene molecule is trigonal planar. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. No lone pair is present on the central or outer atom in the lewis structure of ethene.
Subtract step 3 number from step 1. You can see the lewis structure of C 2 H 2 in above figure and you can see it is a simple structure. Draw the Lewis structure of C2H4.
There are no lone pairs on carbon or hydrogen atoms. From the Lewis structure we can see that the carbon in CO2 must make 2 sigma bonds and it has no lone pairs. When you look at the lewis dot structure of this molecule there are no lone pairs of electrons in its structure.
Polar protic vs polar aprotic vs nonpolar. Alternatively a dot method can be used to draw the lewis structure. There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell.
This atom will be 2sp hybridized with remaining 2px and 2py atomic orbitals. 4 C-H bonds 4 shared pairs of electrons. The structure also has lone pairs of electrons on each Hand lone pair on each C.
C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. There are two triangles overlapping each other as we can see in the diagram. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells.
Find the number of nonbonding lone pairs e-. Calculate the total valence electrons in the molecule. Best Lewis structure 3 points.
Elements in the same group. Which atom is more electronegative bromine. In this tutorial we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1. 15 points for a structure that is plausible but not best bond The Lewis structure has a single double or triple between the carbon atoms. Hence there are two pairs of shared electrons in CC.
Ethane is an organic compound with a chemical formula of C2H6. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. How many lone pairs are there.
C2H6 lewis structure. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. How many lone pairs are there.
Therefore there cannot be more than one stable resonance structure for C 2 H 4. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Use information from step 4 and 5 to draw the lewis structure.
Which grouping of elements will have similar Lewis symbols. A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure EtheneFor the C2H4 structure use the periodic table to find the total number of val. Draw the Lewis structure of SF6.
Xenon has 4 bonding electron pairs and 2 nonbonding pairs so the molecular geometry is square planar. CO Lewis Structure In CO Lewis structurethe carbon atom follows the octet rule and the oxygen atom also follows the octet ruleSoCO follows the octet rule for all the atoms. C2H4 have all bond pairs no lone pair.
12e-2 6 bond pairs. Lewis dot structure of C 2 H 4. 12-12 0e-0 lone pairs.
Draw the Lewis structure of NH3. Ethane Hybridization Molecular Geometry and shape. Each oxygen makes 1 sigma bond and also needs 2 orbitals for lone pairs of electrons.

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Books
Ethene C2h4 Lewis Structure Hybridization
5 2 Molecular Shape Chemistry Libretexts
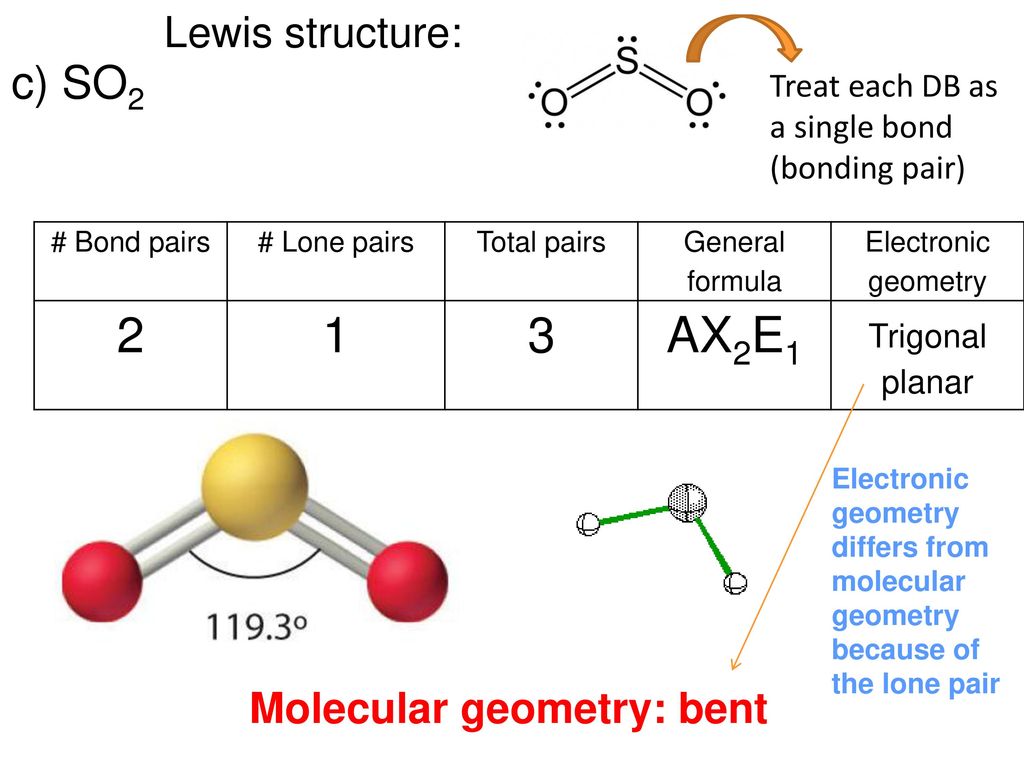
Molecular Geometry Predicted By Vsepr Ppt Download
How Many Lone Pairs Of Electrons Are In 9 Grams Of Water Quora

Molecular Geometry Predicted By Vsepr Ppt Download

Vsepr Theory Rules Application And Geometry Of Ab2 Ab3 And Ab4

6 3 Molecular Shape Introductory Chemistry

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Localized And Delocalized Lone Pairs And Bonds Chemistry Steps
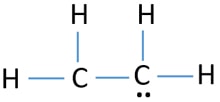
Ethene C2h4 Lewis Structure Hybridization

6 2 Lewis Structures Introductory Chemistry

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Ethene C2h4 Lewis Structure Hybridization
5 2 Molecular Shape Chemistry Libretexts

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist