Lewis Structure Of Pf3 And Its Molecular Shape
The molecule of SF 2 is polar ore nonpolar. The molecule is made up of one Phosphorus atom and three Bromine atoms.

Predict Molecular Geometry Of Pf3 Phosphorus Trifluoride Youtube
Avogadro does not waste his time drawing a Lewis structure before determining the shape of PF3.

Lewis structure of pf3 and its molecular shape. He thinks that the shape of PF3 must be trigonal planar because there are three fluorine atoms bonded to the central. Identify the molecular geometry of SF 2. As a result the shape of the molecule is trigonal pyramidal and it ensures a non zero dipole moment making the PF3 a polar molecule.
Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. Lets count the areas around the phosphorus atom that. Draw a Lewis structure for the molecule.
The molecular geometry of NF3 is a trigonal pyramid and its electron geometry is tetrahedral because nitrogen has Sp³ hybridization with 5 valence electrons in its valence shell and it makes three bond pairs one with each fluorine atom. The phosphorus trifluoride chemical formula is PF3. Saturday March 6 from 34 PM PST.
Like PF3 and PCl3 PBr3 also exhibits the properties of both Lewis Acid and Lewis Base. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition. C r -p -F Determine the total number of electron groups around the.
Species Lewis Structure VSEPR Shape name Molecular Shape name HCN PF3. PF3 has 26 valence electrons. Molecular shape for PF3.
Three single covalent bonds are formed between the phosphorus and fluorine atoms which contributes to the presence of three strong sigma bonds and no pi bonds. PF3 has 26 valence electrons. PBr3 is a chemical formula for Phosphorus Tribromide.
What is the hybridization of central atom. Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. Solution for Formula Lewis structure Molecule or Electron group Molecular Bond Polarity Ion Type geometry geometry angle PF3 H2O PF3.
Draw the Lewis structure for SF 2 showing all lone pairs. Complete the following table Element or Ion Number of protons Number of electrons Number of neutrons 180 36C1 40Ca2 A Draw a Lewis structure for each of the following substances and give its VSEPR shape its molecular shapes and polarity. A step-by-step explanation of how to draw the PF3 Lewis Dot Structure Phosphorous trifluorideFor the PF3 structure use the periodic table to find the tota.
It is a colourless liquid with a pungent odour. VESPR stands for valence shell electron pair repulsion. What is the approximate bond angel in SF 2.
Lewis structure of Phosphorus Trifluoride PF3 The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. Sp 3 d 2 2. See full answer below.
The Lewis structure of PF3 shows that the central phosphorus atom has nonbonding and bonding electron pairs. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule PF_3 because of its AX_3E status. PF3 is a polar molecule.
Due to its original pyramidal shape the PF3 molecule turns out to be polar. In chemistry molecular geometry refers to how the atoms in a molecule are arranged in 3D. Learn vocabulary terms and more with flashcards games and other study tools.
Here in this post we described. Draw a Lewis structure for the molecule. The Lewis structure of PF 3 is.
This gives us an idea of the molecules shape as well as the bond angles torsional. Phosphorus trifluoride is a toxic substance and is odorless and colorless in appearance. Molecular shape for H2S.
495 7836 Views. The molecule of PF 3 is polar or nonpolar. The VSEPR shape of the molecule PF_3 is trigonal pyrimidal.
Click to see full answer. Start studying Lewis Dot Structure of Molecules Lewis Structures VSEPR Shapes. PF 3 has a trigonal pyramidal molecular geometry.
Bond angle 1095 Because of the lone pair the bond angle will be less than 1095. Drawing PF3 Lewis Structure is very easy to by using the following method. Draw a Lewis structure for the molecule.
Phosphorus and fluorine have different electronegativity and the PF3 molecule also contains a lone pair. PF3 has 26 valence electrons. PBr3 Lewis Structure Molecular Geometry Hybridization and Polarity.

Answer The Molecular Geometry Of Pf3 Is B Clutch Prep

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

9 3 Drawing Lewis Structures Chemistry Libretexts

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
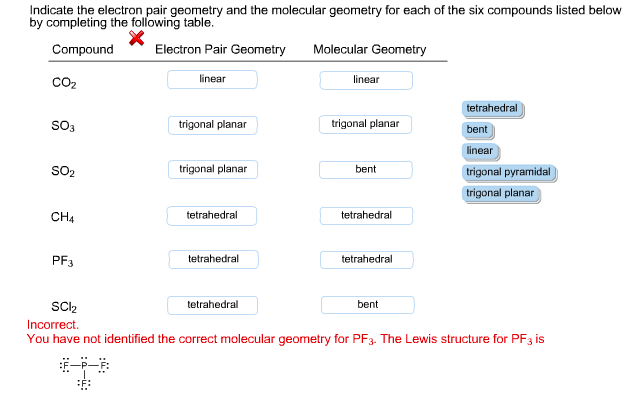
Indicate The Electron Pair Geometry And The Molecular Chegg Com

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
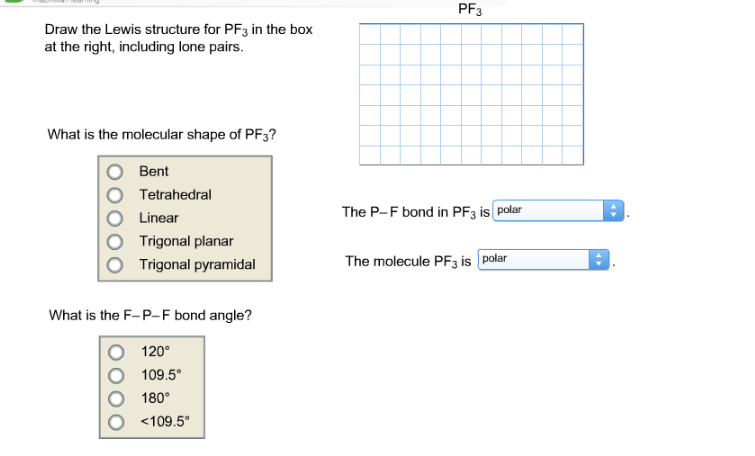
Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com
Pf3 Molecular Geometry Shape And Bond Angles دیدئو Dideo
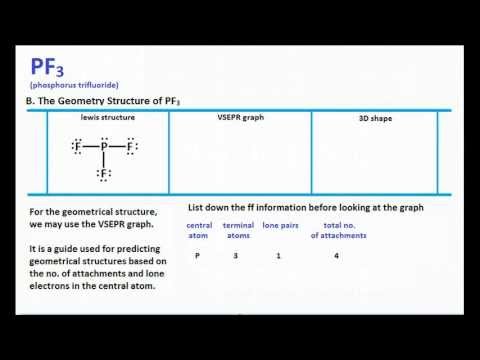
Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Draw The Lewis Structure For Pf3 In The Box At Chegg Com

Lewis Structure For Pf3 Learn Lif Co Id
Draw The Lewis Dot Structure For Ch Cl Determine The Chegg Com

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Pf3 Molecular Geometry Shape And Bond Angles Youtube

Pf3 Molecular Geometry Shape And Bond Angles Youtube

What Is The Molecular Geometry Of Pf3 Study Com

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
7 1 Point Given The Lewis Structure Of Pf3 Below Chegg Com

