Brf3 Lewis Structure Lone Pairs
A step-by-step explanation of how to draw the BrF3 Lewis Dot Structure Boron trifluoride For the BrF3 structure use the periodic table to find the total n. Bromine trifluoride BrF3 molecule is bent in structure due to the presence of two lone pairs on bromine atom.
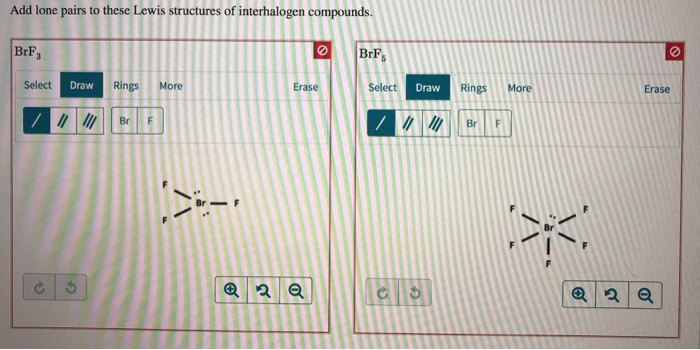
Add Lone Pairs To These Lewis Structures Of Chegg Com
The chlorite ion ClO 2 contains 19 7 from the Cl and 6 from each of the two O atoms 1 20 electrons.
Brf3 lewis structure lone pairs. BrF3 does not follow the octet rule. As three electrons out of seven form a bond with the valence electrons in the Fluorine atom there are four nonbonding electrons on the central atom of BrF3. BF3 Lewis Structure Molecular Geometry Hybridization and Polarity.
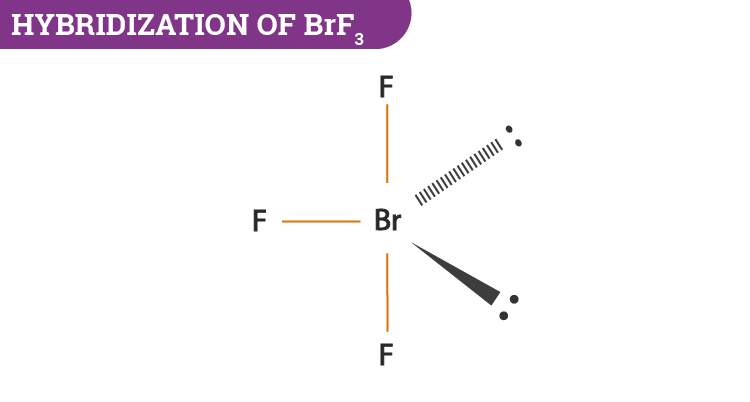
Hence the hybridization would be sp3d. A video explanation of how to draw the Lewis Dot Structure for Bromine Trifluoride along with information about the compound including Formal Charges Pola. 2What is the Lewis structure for BrF3.
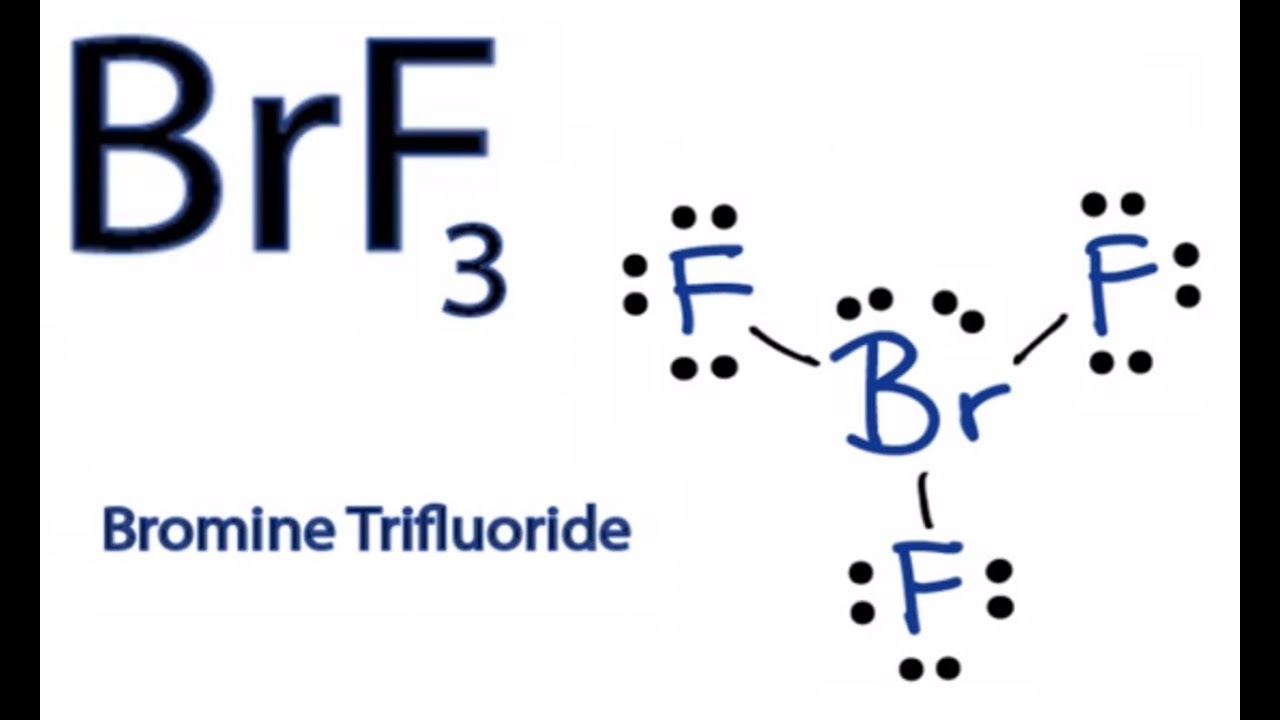
BrF3 has a Bent T shaped structure with Br as a central atom bonded with three F atoms three bond pairs and two lone pairs. Bromine trifluoride chemical formula is BrF3. The outer shell of the Bromine molecule has seven valence electrons and three of them bond with the three fluorine atoms.
For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule. One electron is added because the entire molecule has a -1 charge. There are a total of 28 valence electrons for the BrF 3 Lewis structure.
Draw this VSEPR structure next to the Lewis structure. In its outermost shell BrF3 has seven electrons. The BrF3 molecular geometry is in T-shaped or Trigonal Bipyramidal with Bromine as the central atom.
Valence electrons so the Lewis structure will have a total of 28 electrons or 14 electron pairs. Draw this VSEPR structure next to the Lewis structure. Hence there are two lone pairs of electrons or four nonbonding electrons on the central atom Bromine of BrF3.
10Draw the Lewis structure for NH3 b What is the electronic geometry of this molecule look at atoms and lone pairs. Draw the Lewis structure for BrF3 b What is the electronic geometry of this molecule look at atoms and lone pairs. Bromine Flouride has 28 valence electrons which result in forming three bonds in the molecule and two lone pairs of electrons on the Bromine.
The electron pairs hybridization value is equal to 5 resulting in sp 3 d hybrid orbitals. For the BrCl 3 Lewis structure youll need to put more than eight valence electrons on the Bromine atom. Br is the central atom connected to each F atom by a single.
Draw the Lewis structure of BrF3 and include the lone pairs. Such compounds are known as inorganic compounds as they are not the organic ones because of lacking Carbon. Hence the number of lone pairs on the central atom of the compound boron trichloride will be 0.
By signing up youll get thousands of step-by-step solutions to your. Boron trifluoride is the inorganic compound and its formula is BF3. In the Lewis structure for BrCl 3 there are a total of 28 valence electrons.
After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets. Drawing BrF3 Lewis Structure is very easy to by using the following method. Because Br is in period 4 it has an n value of n 4.
It contains two lone pairs and three BrF covalent bonds after bond formation. Draw the Lewis structure for the BrF3 molecule. Therefore l can be equal to 0 1 2 or 3.
The hybridization of the central atom is sp3d but to minimize the repulsion between the lone pairs the shape. B In the compound BrF3 B r F 3 bromine trifluoride the central atom bromine has 7 valence. What is the structure of XeF2.
The shape is affected because of the three bonded pairs of electrons and two lone pairs of electrons. The lewis structure of BrF3 permits Br to have 10 valences electrons which is above the octet rule of 8 valence electrons because it is in period 3 or higher of the periodic table. XeF2 structure features two covalent bonds between one xenon atom and two fluorine atoms.
Steric Number stands for the sum of the number of bonded electrons and the lone pair on the central atom. Steric number in BrF3 32 5. We have two lone pairs on the Bromine atom an exception to the octet rule.
The steric number is an important term here which we need to find out for any VSEPR calculation. Bromine is the least electronegative atom in the BrF 3 Lewis structure and therefore goes at the center of the. Secondly what is the Lewis structure for ClO.
Molecule by placing atoms on the grid and connecting them with Include all lone pairs of electrons. Bromine trifluoride is a compound that contains one bromine atom and three fluorine atoms. You may have heard about the chemical compound that lacks C-H bonds.
The l value of 2 creates a d-orbital and the l value of 3 creates the f-orbital. Here in this post we described step.

Solution Add Lone Pairs To These Lewis S Chemistry

Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules
What Is The Hybridization Of Brf3 Quora
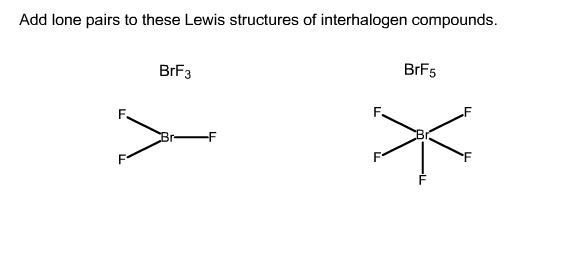
Add Lone Pairs To These Lewis Structures Of Chegg Com
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Solution Add Lone Pairs To These Lewis S Chemistry

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

5 Draw The Most Appropriate Lewis Structure S For Brf3 Wh Clutch Prep

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

How Many Lone Pairs Are On The Central Atom Of Brf3 Express Clutch Prep
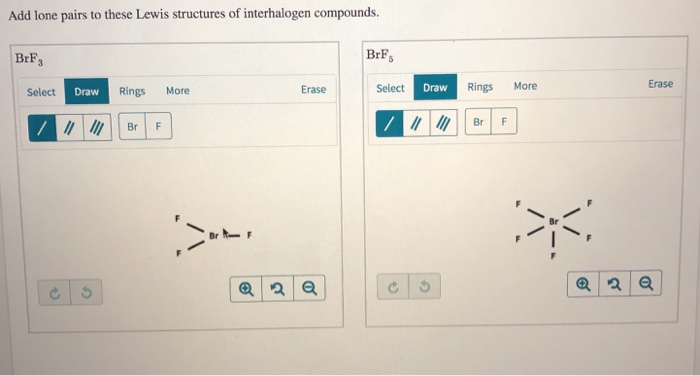
Add Lone Pairs To These Lewis Structures Of Chegg Com
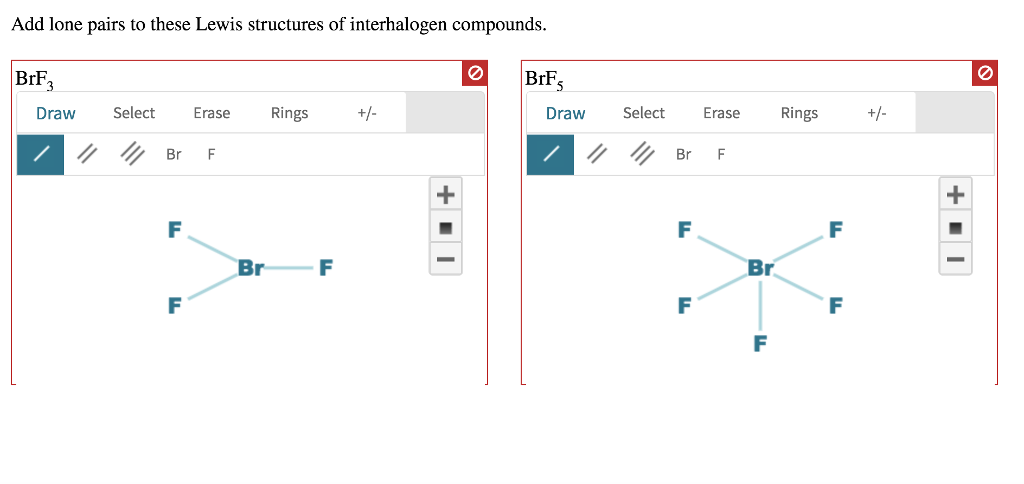
Add Lone Pairs To These Lewis Structures Of Chegg Com

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
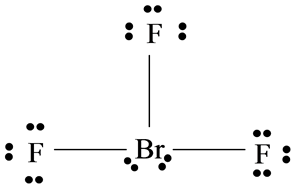
Solved Chapter 22 Problem 21e Solution Selected Solutions Manual General Chemistry 10th Edition Chegg Com