Lone Pairs In Xef4
The central atom has no lone pair and there are four bond pairs. XeF4 is stable when subjected to normal pressure and temperature.

Molecular Geometry Of Xef4 Youtube
Therefore XeF4 molecular geometry is square planar.
Lone pairs in xef4. The XeF4 xenon tetrafluoride molecule is hypervalent with six electron pairs around the central xenon Xe atom. The total number of electrons around the central atom S is eight which gives four electron pairs. Just because an atom has 4 attachments does not make it tetrahedral.
Xenon tetrafluoride XeF4 is a square planar non-polar molecule. So there are 12 lone pairs on F atoms. XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other.
The maximum number of 90 angles between bond pair of electrons is observed in. The sublimation of the compound occurs at 1157C. 36-8 28e-14 lone pairs Use information from step 4 and 5 to draw the lewis structure.
The molecule has a grand total of 36 electrons. Each fluorine atom has three lone pairs. The bond angles are 90 or 180.
XeF4 has a square planar molecular shape so all the bond are in the equatorial position. Subtract step 3 number from step 1. SF IFS 13 H30 trigonal trigonal planar NOR Polar planar No CO3.
How many lone pairs are in SiCl4. In XeF2 XeF4 XeF6 the number of lone pairs on Xe are respectively. Hence there are a total of 36 valence electrons in XeF4.
As the Noof electrons in outermost shell of. The same study is backed up by VSEPR theory as there are two lone pairs in xenon. If you look at the Lewis Dot structure of XeF4 you can see that theres 36 total electrons.
Likewise How do you know if its polar or non polar. However when it comes in contact with water it reacts with it and releases xenon gas molecular oxygen and xenon fluoride. Yes you are correct XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral.
Since there are 4 F atoms and each F atom has 3 lone pairs total lone pairs are 34 12. In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other. Iodine have two lone pair and four bond pair two lone pairs one above and below the plane on the one-axis ie.
In XeF 4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs around xenon atom. A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. Lone pairs occupy opposite electron domains to minimise repulsion forces all the four fluorine are opposite to each other and place in.
The Xenon atom has 4 bonding pairs of electrons and 2 lone non-bonding pairs of electro. The number of lone pairs of electrons on Xe in X eF 2X eF 4 and X eF 6 are respectively are 3 2 and 1. But well only 4 electrons of Fluorine have to be bonded with Xe in order to obtain XeF4 which leaves out 4 electrons of Xe unbonded.
PE5 SF4 CIF3 XeF2 XeF4 Formula Total Domains Lewis Structure Lone Pairs on Central Atom Electron Domain Geometry Polar Nonpolar Molecule Molecular Geometry Polar Bonds Present. And since we know that 1 lp2electrons Therefore XeF4 will have 2 lone pairs. In XeF4 two lone pairs lp are present.
Lewis dot structure of XeF4 Alternatively a dot method can be used to draw the lewis structure. What is shape of XeF4 xeo3. Therefore XeF4 molecular geometry is square planar.
These pairs adopt an octahedral arrangement. In this tutorial we will learn how to draw lewis structure of XeF 4 step by step. Each fluorine atom has three lone pairs.
XeF4 consists of two lone pair electrons. The geometry of XeF4 is a square planar with symmetric electron reigon distribution. 3 lone pairs on each chlorine 2 lone pairs on the central iodine and 8 bonding electrons.
The central atom in XeF4 is surrounded by a3 single bonds 1 double bond and no lone pairs of electrons. AskedMar 31 2018in Chemistryby paayal147kpoints. Four of the pairs are bonding pairs and two are lone pairs.

How Many Numbers Of Lone Pair Is Present In Xef4 Quora

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
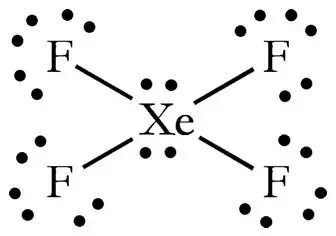
How Many Numbers Of Lone Pair Is Present In Xef4 Quora
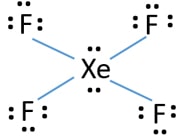
Xef4 Xenon Tetrafluoride Lewis Structure
Why Isn T Xef4 Sea Saw Shaped Physics Forums
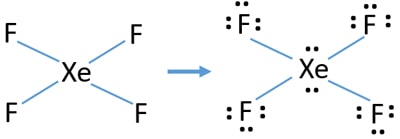
Xef4 Xenon Tetrafluoride Lewis Structure

Number Of Lone Pairs And Bonding Pairs For Xef4 Xenon Tetrafluoride Youtube

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube
How Many Numbers Of Lone Pair Is Present In Xef4 Quora

Lewis Dot Structures Of Ionic And Covalent Compounds Persuasive Writing Prompts Covalent Bonding Worksheet Chemistry Worksheets

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube
How Many Numbers Of Lone Pair Is Present In Xef4 Quora

Lewis Structure Vsepr For Xef4 Youtube
What Is The Vsepr Structure Of Xef4 Quora
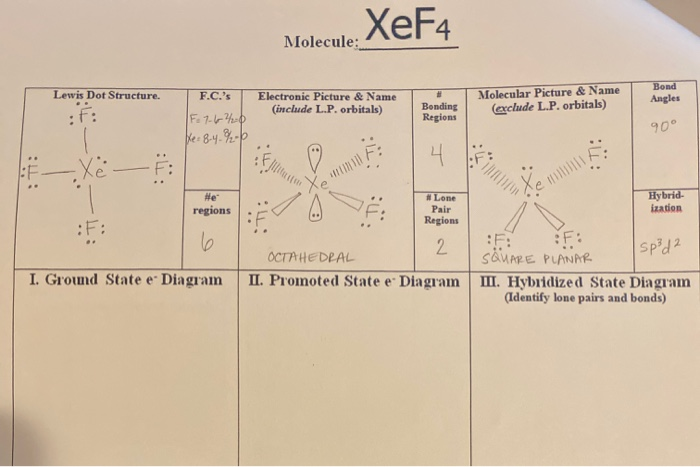
Xef4 Molecule Lewis Dot Structure F C S Electronic Chegg Com

Complete The Hybridization And Bonding Scheme For Xef4 Brainly Com

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
Lewis Structure Of Xef4 Biochemhelp
