Hno3 Lewis Structure Shape
Number of valence electrons b. The N atom has steric number SN 3.

Hno3 Lewis Structure Nitric Acid Youtube
This is a pattern seen with many acids.

Hno3 lewis structure shape. B write the electron group geometry c write the molecular geometry d determine if the molecule is. So according to the VSEPR chart H3O has trigonal pyramid as its molecular shape and tetrahedral as its electron geometry. There are three single bonds and one lone pair of electrons in NH3 molecule.
HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. The molecular shape of H3O is a trigonal pyramid and electronic geometry is tetrahedral. The HNO3 Lewis structure has 24 valence electrons.
The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. The shape around the Nitrogen 5 hastrigonala geometry and the shape around the oxygen is a bent or V shape. For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule.
The central carbon atom is still joined to two other atoms. The shape is distorted because of the lone pairs of electrons. The electron geometry is trigonal planar.
Hydrogen Cyanide is a colorless flammable and poisonous chemical liquid. We write the Lewis structure. H O 3 P O The given Lewis structure distributes 5 bonding electron pairs about the central phosphorus atom.
After determining how many valence electrons there are in HNO3 place them around the central atom to complete the. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. This pair exerts repulsive forces on the bonding pairs of electrons.
From the above chart we can see that hydronium ion is a AX3E type molecule A central atom X bonded atom E lone pair on A. Be sure to use the number of available valence electrons you found earlier. Lewis structure of nitric acid.
Molecule Valence E- Lewis Structure Molecular Shape. If we look at the Lewis molecular structure of HNO3 we can see H has one N has five while O atom has six valence electrons. The N atom is sp² hybridized.
16 bonding and non-bonding electron pairs. The HNO 3 Lewis structure is easier to think of if you consider it NO 3 with an H bonded to one of the oxygen atoms. Represented by the chemical formula HCN is one of those molecules that has an interesting Lewis structure.
Check the formal charges to. Identify the central atom s in all of these structures Hint. Another way of looking at molecular geometries is through the AXE method of electron counting.
The oxygen atoms will also have two p orbitals which will accommodate lone pair of electrons. Hocl molecular shape - You can only upload photos smaller than MB. Drawing the Lewis Structure for HNO After determining how many valence electrons there are in HNO3 place them around the central atom to complete the octets.
HCN Lewis Structure Molecular Geometry Shape and Polarity. This liquid is used in electroplating mining and as a precursor for several compounds. Space Filling Model A and Ball and Stick Model B.
3 H P 5 24 O 32 electrons ie. Formal Charge Polar Molecule. The Lewis structure and the VSEPR valence shell electron pair repulsion theory.
Draw the Lewis dot structure for eqHNO_3 eq and provide the following information. For the lewis structure of NO3- N is the central atom and there are 3 oxygens surrounding it. Also what is the shape of Hocl.
If we look at the shape of the HNO3 molecule it has a trigonal planar shape. The Lewis structure of HNO₃ shows that it is a resonance hybrid of two structures. Draw the Lewis structures of the HNO2 HNO3 H2S PH3 CH3F and HCCH acetylene.
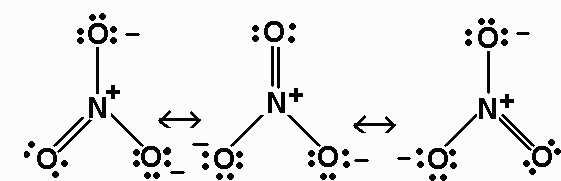
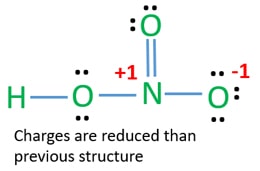
HNO 3 Nitric acid Lewis Structure. Drawing the Lewis Structure for HNO 3. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom.
Postby Clarissa Cabil 1I Tue Nov 20 2018 1110 pm. HNO3 Quick Time Movie. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms.
The molecular geometry of any molecule is determined by two things ie. One of the oxygens has a double bond with N and the other two oxygens have a single bond with N because nitrogen is in period 2 so it cannot have more than 4 bonds. Resonance SO42- NO3- CO2 SCN- HNO3 CO32- PO3-.
In HNO 3 Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. The first two have an NOH and a list how many electron groups are around the atom.

Indicate The Electronic Geometry And The Molecular Geometry Clutch Prep

Hno2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

How Is The Lewis Dot Structure For Hno3 Determined Quora

1 Draw The Lewis Structure Of Nitric Acid Hno 3 That Minimizes Formal Charges Assign Lone Pairs Radical Electrons And Atomic Charges Where Appropriate 2 Calculate The Electrons Required Er Study Com

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

How To Determine The Molecular Geometry Of Hno3 Quora
7 6 Molecular Structure And Polarity Chemistry

How Is The Lewis Dot Structure For Nitric Acid Determined Quora

The Lewis Structure Of Hno3 Chemistry Stack Exchange

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Hno3 Nitric Acid Lewis Structure

What Is The Lewis Structure Of Ni3 Study Com
In Lewis Structure Of Hno3 Does Nitrogen Share Two Electrons With Two Of The Oxygens Do Those Oxygens Share Or Not Share Electrons With The Nitrogen Quora

What Is The Lewis Structure For H3bo3 Study Com
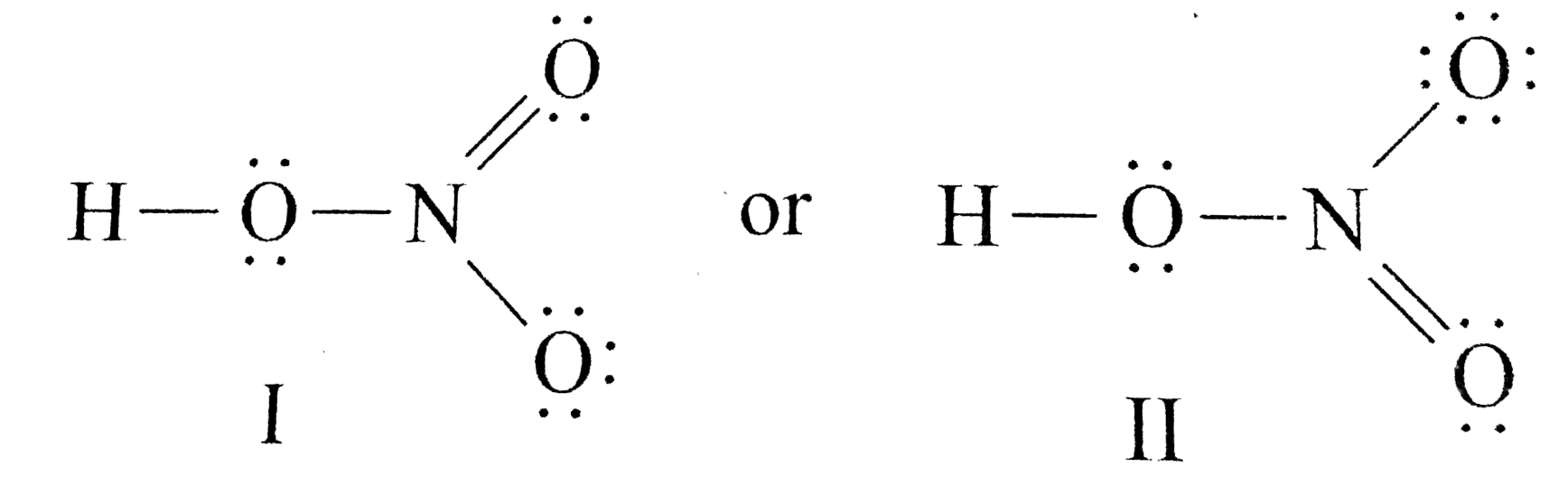
Draw The Lewis Structure Of Nitric Acid Hno 3
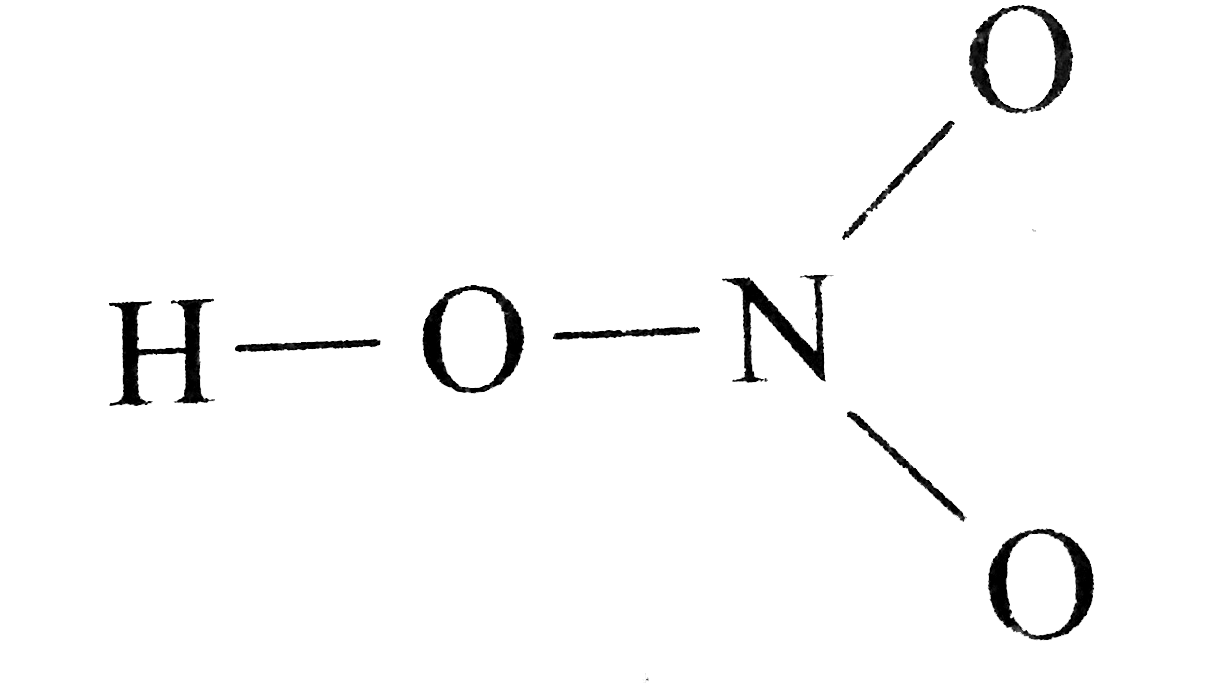
Draw The Lewis Structure Of Nitric Acid Hno 3

Draw A Lewis Structure For Nitric Acid The Hydrogen Atom Is Attached To One Of The Oxygen Atoms Brainly Com