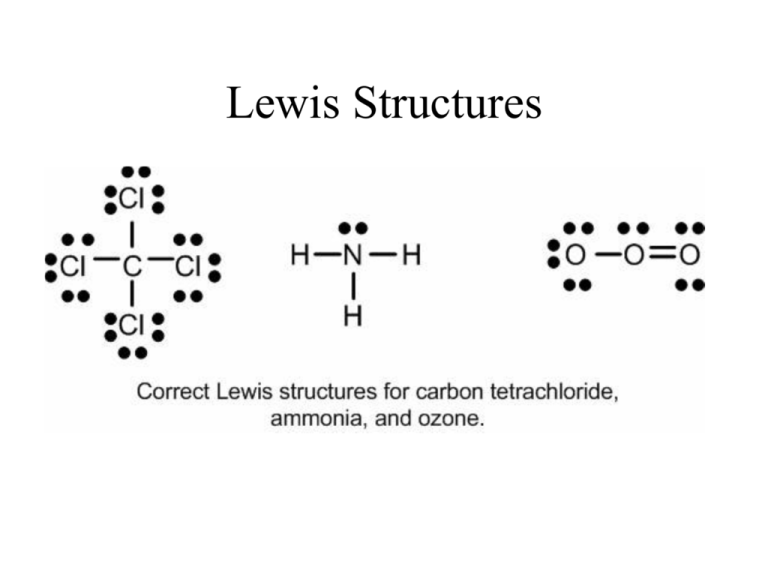
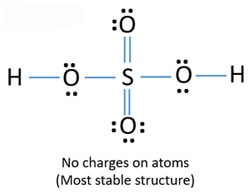
Correct Lewis Structure For H2so4
While PM7 isnt a highly accurate method the difference in stability isnt close. Hence 322 given the result 16.
![]()
Draw A Lewis Structure For Co32 And Answer The Following Questions A What Is The Number Of Lone Pairs B What Is The Number Of Single Bonds C What Is The Number
Weve used 10 valence electrons.

Correct lewis structure for h2so4. What is the correct Lewis Structure for Sulfuric Acid H2SO4 that minimizes formal charge. Lewis Dot Structure Of H2so4 Sulfuric Acid Youtube. H----H O0 0.
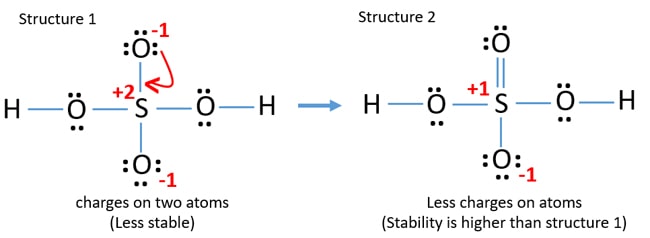
Oxygen and Sulfur atoms possess six electrons in their valence shells. When we have an H or H2 in front of a polyatomic molecule like CO3. There are no charges at any atom.
The lowest energy geometry is the traditional H X 2 S O X 4 Lewis structure estimated at Δ H f 0 -17788 kcalmol. Lewis Dot Structure Of H2so4 Brainly In Hiiiiii a step by step explanation of how to draw the h2so4 lewis structure sulfuric acidwhen we have an hor h2in front of a polyatomic molecule like co3so4no2etc we know that its an acid. I also go over hybridization shape and bond angles.
There are three single bonds in the molecule. This means that the hydrogen atoms will be attached to the outside of the oxygen molecules. One hydrogen atom is joint to phosphorous atom and remaining hydrogen atoms are joint to.
This chemistry video tutorial explains how to draw the lewis structure of H2SO4 Sulfuric AcidMy Website. It has a molecular weight of 98079 gmol. Note lone pairs can be converted into bonds until we get a stable structure.
H 2 so 4 step 7 picture so far. 6- - OH 0 6- Lo. H2so4 works as an oxidizing and dehydrating agent.
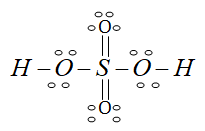
This is the h2so4 lewis structure. Sulfur has 8 valence electrons each of the Oxygens has an octet and the Hydrogen here it has 2 so its outer shell is full. H----H O0 0.
In the h2so4 structure lewis sera is the least electronic atom and goes to the center of lewiss structure. 6- - OH 0 6- Lo. Answer to Identify the correct Lewis structure for H2SO4.
That means youre going to have an acid and that these hs are going to be attached to the outside of the oxygens. Lewis Dot Structure of H2SO4 Step 1 H2SO4 Valence Electrons. O 10 O 12.
Lewis structure of sulfuric acid is drawn step by step in this tutorial. I quickly take you through how to draw the Lewis Structure of H2SO4 Sulfuric Acid. S does not follow the octet rule.
Drawing the correct lewis structure is important to draw resonance structures correctly Total number of electrons of the valance shells of H 2 SO 4 Both Sulfur and oxygen atoms are located at VIA group in the periodic table. Draw the Lewis dot structure of the following. Demonstration Video View Notes.
A step-by-step explanation of how to draw the H2SO4 Lewis Structure Sulfuric Acid. H2so4 is a chemical formula of sulfuric acid which is commonly known as oil of vitriol. 0 0 -.
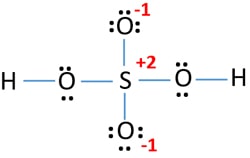
For the H2SO4 molecule sulfur has the highest. This looks like a really good Lewis structure for HSO4- but Sulfur is in period 3 on the periodic table so its a good idea to check the formal charges on each atom to make sure theyre as close to zero as possible. C O 3 2 H C l O 4 H N O 3.
H2so4 lewis structure molecular geometry and hybridization. We have a total of 26 valence electrons for the H2SO4 Lewis structure. H2so4 lewis structure.
It means the total pairs of electrons are 16. Entonces tendremos que poner un grupo oxidrilo por cada hidrógeno. The key to.
Its a mineral acid composed of elements like oxygen hydrogen and sulfur. The key to understanding this lewis structure is that you have these hs in front and then you have this polyatomic ion. Draw The Lewis Structure For H2so4 lewis structure draw lewis structures acid sulfuric electrons valence structure resonance draw octet rule many bonded shapes each brainly lewis structure drawing begingroup stack lewis acid sulfuric draw structures bonded.
Well put 2 electrons between atoms to form the chemical bonds there. It has a molecular weight of 98079 g mol. H2SO4 works as an oxidizing and dehydrating agent.
So you have 10 12 24 and then back to the center 26. Identify The Correct Lewis Structure For H2SO4. Lewis structure of phosphoric acid contains -1 charge on one oxygen atom and 1 charge on phosphorous atom.
Then well fill the octets on the Oxygens. 0 0 -. So weve used all 26 valence electrons and everything in the structure in the H2SO4 Lewis structure has a full outer shell.
This is the h2so4 lewis structure. Formal charge on an atom in a Lewis structure total number of valence electrons in free atom total number of non-bonding lone pairs electrons 12 total number of bonding or shared electrons. Lewis dot of the sulfuric acid.
H 2 SO 4 Step 8 Picture so Far.
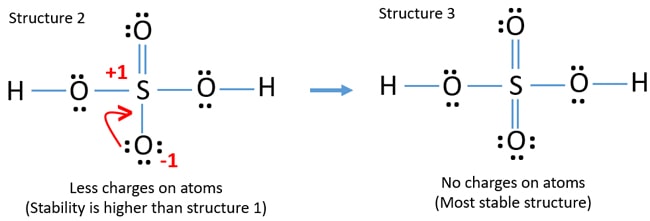
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

H2so4 Lewis Structure Sulfuric Acid Youtube

H2so4 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Draw And Explain The Lewis Structure For Sulfuric Acid Study Com

H2so4 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Diagram Lewis Diagram So4 Full Version Hd Quality Diagram So4 Snadiagram Bmwe21fansclub It
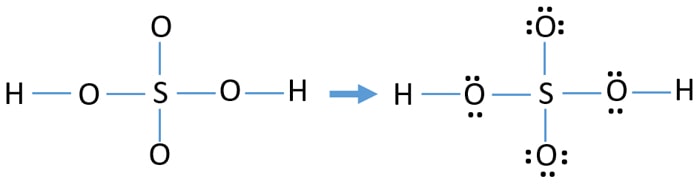
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

Lewis Dot Structure Of H2so4 Brainly In
Draw H2so4 Lewis Dot Structure Class 11 Chemistry Cbse

H2so4 Lewis Structure Sulfuric Acid Youtube
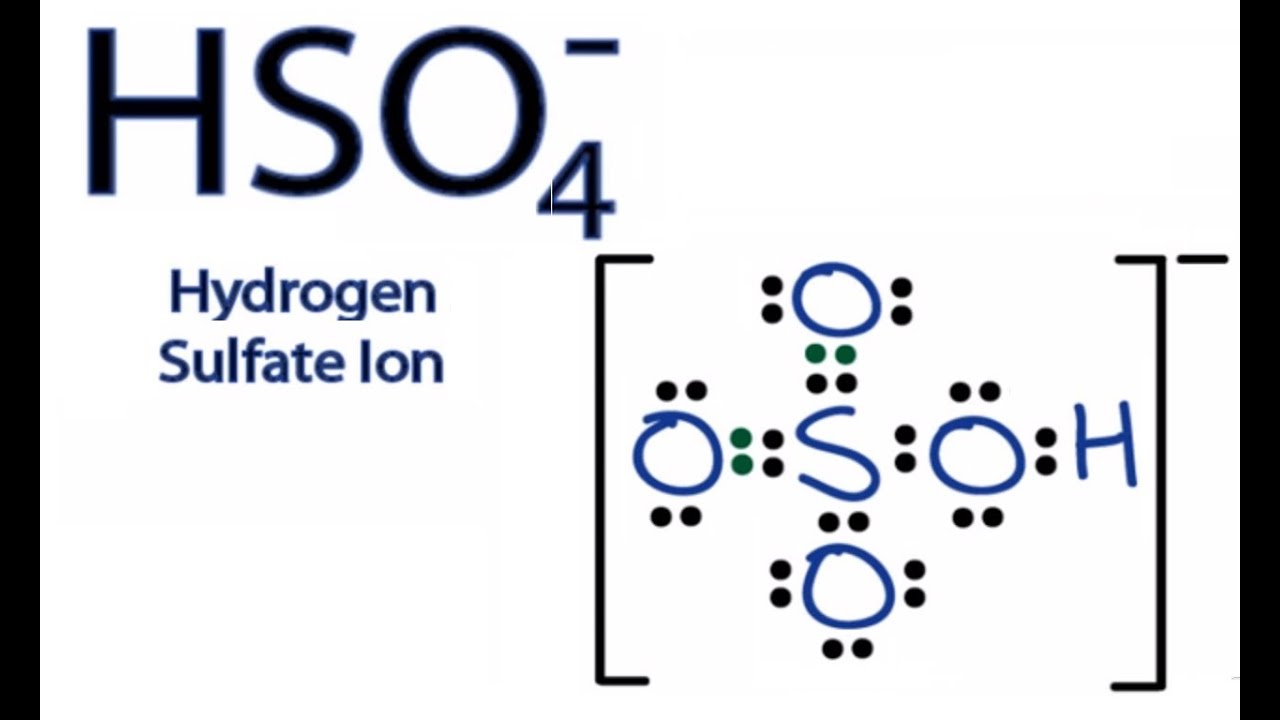
Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube
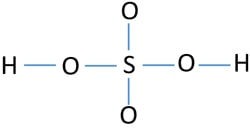
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

H3po4 Lewis Structure How To Draw The Lewis Structure For H3po4 Youtube
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

There Are Many Lewis Structures You Could Draw For Sulfuric Acid H2so4 Each H Is Bonded To An O Brainly Com