Xef4 Lewis Structure Lone Pairs
One lone pair of electrons on the sulfur atom in the bipyramidal geometry of SCl4 molecule. Elements in the first 2 periods of the Periodic Table do not have access to the d sublevel and must adhere to the octet or duet H and He rule.

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Step method to draw lewis structure for XeF4 This molecules is an example of expanded octet Step 1.
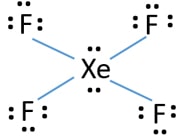
Xef4 lewis structure lone pairs. The maximum number of 90 angles between bond pair of electrons is observed in. Write the Lewis structure for XeF4. Bromine gets 12 electrons in order to make 5 bonds with surrounding atoms.
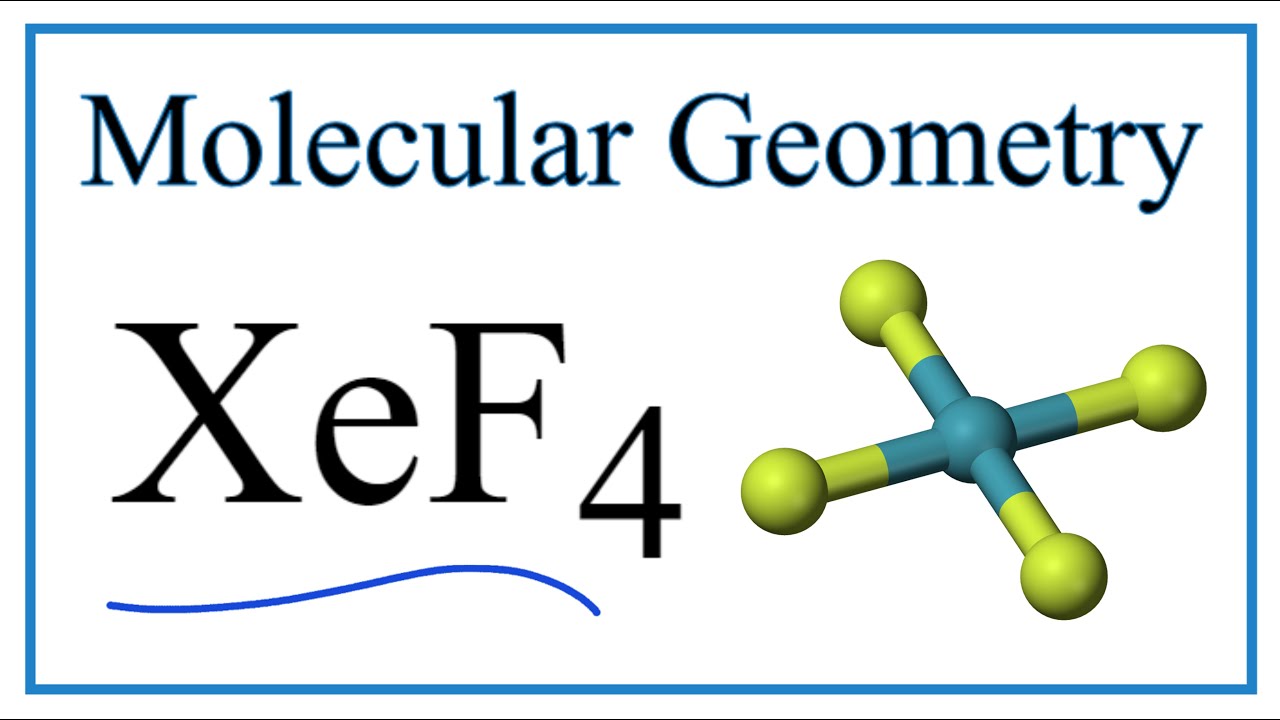
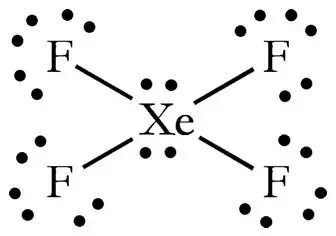
Include all lone pairs of electrons. Therefore XeF4 molecular geometry is square planar. Since there are 4 F atoms and each F atom has 3 lone pairs total lone pairs are 34 12.
So there are 12 lone pairs on F atoms. In XeF 4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs.
So far weve used 34 of the SCl4 Lewis structures total 34 outermost valence shell electrons. It is a powerful fluorinating as well as an oxidizing agent. Out of these compounds XeF2 is the most.
The total number of electrons around the central atom S is eight which gives four electron pairs. Draw the molecule by placing atoms on the canvas and connecting them with bonds. XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other.
Its chemical equation could simply be written as. Xef4 has 2 lone pairs on xethe lone pairs are opposite to each other and their attractionrepulsion is as the molecule is symmetrical all forces within the molecule cancel out each other. PE5 SF4 CIF3 XeF2 XeF4 Formula Total Domains Lewis Structure Lone Pairs on Central Atom Electron Domain Geometry Polar Nonpolar Molecule Molecular Geometry Polar Bonds Present.
Find valence e- for all atoms. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. In this tutorial we will learn how to draw lewis structure of XeF 4 step by step.
So in total there are 2 1Xe 3 4F 14 lone pairs of electrons. There are also 3 lone pairs on each F atom. Just because an atom has 4 attachments does not make it tetrahedral.
In this process elemental fluorine supposedly oxidizes xenon under some specific conditions of. Yes you are correct XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. Complete the middle sulfur atom stability and if necessary apply a covalent bond.
According to the VSEPR theory the shape of the molecule is predicted by the total number of electron pairs lone pairs bond pairs in the valence shell of the central Xe atom. The Lewis structure for XeF4 has a total of 36 valence electrons. XeF 4 is d 2 sp 3 hybridized and contains 2 lone pair and 4 bonding pairs of valence electrons around the Xenon.
XeF4 Lewis Structure Molecular Geometry Hybridization and MO Diagram. The central atom in XeF4 is surrounded by a3 single bonds 1 double bond and no lone pairs of electrons. Apart from XeF2 there are other Xenon compounds such as XeF4 Xenon Tetrafluoride and XeF6 Xenon Hexafluoride.
SF IFS 13 H30 trigonal trigonal planar NOR Polar planar No CO3. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. The VSEPR predicts the Square Planar shape.
In XeF4 there are 2 lone pairs on the Xe atom. The geometry of XeF4 is a square planar with symmetric electron reigon distribution. Just because an atom has 4 attachments does not make it tetrahedral.
The central sulfur atom undergoes extra octet stability. 3 O- X C-0. So the hybridization is sp3d2 and it has 2 lone pairs the shape of XeF4 is square planar.
This chemical compound is formed when xenon reacts with fluorine. Find octet e- for each atom and add them together. Each fluorine atom has three lone pairs.
Xe 2F2 XeF4. Xef2 Lewis Structure Polarity Hybridization and shape. XeF4 has a square planar molecular shape so all the bond are in the equatorial position.
When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. Subtract step 3 number from step 1. XeF4 is the chemical formula of the compound Xenon Tetrafluoride.
Yes you are correct XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. What is shape of XeF4 xeo3.
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube
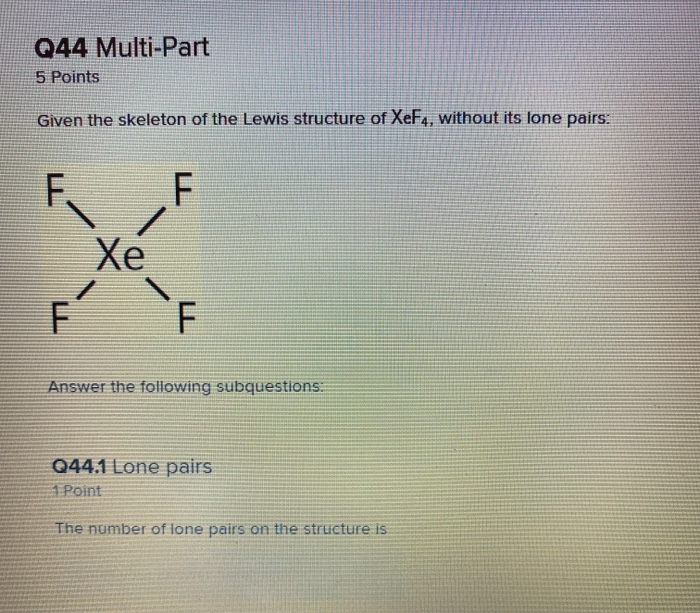
Q44 Multi Part 5 Points Given The Skeleton Of The Chegg Com

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube
How Can The Lewis Structure For Xef4 Be Determined Quora

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

In The Best Lewis Structure For Xef4 What Is The Formal Charge On The F Atom A 1 B 0 C 1 D 2 Study Com

Molecular Geometry Predicted By Vsepr Ppt Download

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Number Of Lone Pairs And Bonding Pairs For Xef4 Xenon Tetrafluoride Youtube
How Can The Lewis Structure For Xef4 Be Determined Quora
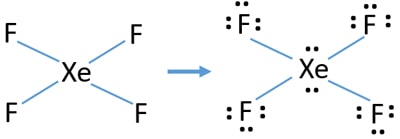
Xef4 Xenon Tetrafluoride Lewis Structure
Lewis Structure Of Xef4 Biochemhelp

How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
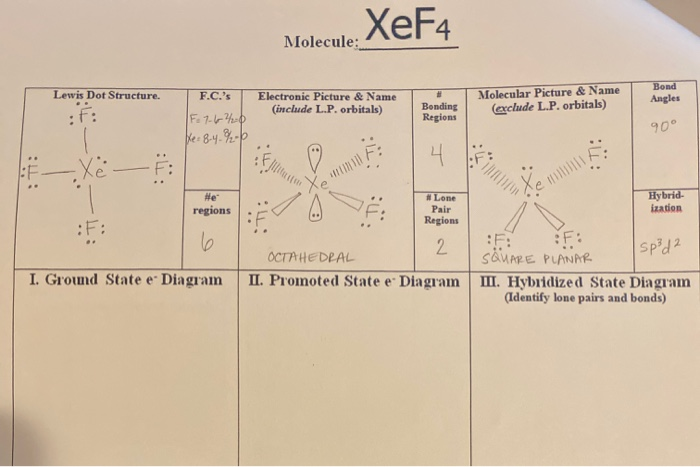
Xef4 Molecule Lewis Dot Structure F C S Electronic Chegg Com

Why Does The Lewis Structure Of Xef4 Not F Clutch Prep

Xef4 Xenon Tetrafluoride Lewis Structure
What Is The Vsepr Structure Of Xef4 Quora