Lewis Structure H3o+
H3O is an important compound in Acid. Assume that bonding follows the octet rule.

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
The central atom for H 3 O is O since Hs cant be a central atom.

Lewis structure h3o+. Lone Pair e- 8 8 0. Bond Pair e- 8. O has 2 bonding electrons and 2 lone pairs therefore a neutral oxygen.
A NH3 b HCO3-c CO3 2-d ClF3 E NF3. From the above chart we will see that hydronium ion is a AX3E kind molecule A central atom X bonded atom E lone pair on A. The molecular shape of H3O is a trigonal pyramid and electronic geometry is tetrahedral.
The Lewis structure of this molecule has to be known first to better understand its physical and chemical properties. Youll find the correct answer below. General Organic and Biochemistry with Connect Plus Access Card 7th Edition Edit edition Solutions for Chapter 3 Problem 10PP.
Therefore we only have 8 valence electrons for the H3O Lewis structure. The molecular shape of H3O is a trigonal pyramid and electronic geometry is tetrahedral. Describe the bonding in this species.
The Correct Answer is. Show all unshared pairs and the formal charges if any. In H3O ion there is no vacant orbital octet of oxygen is fulfilled and duplet of hydrogen atoms are also fulfilled.
Oxonium is an oxygen hydride and an onium cation. The structure will help in understanding the arrangement of atoms bond formation and shape of the molecule. I quickly take you through how to draw the Lewis Structure of hydronium ion H3O.
Correct answer to the question Draw the Lewis structure for the polyatomic hydronium H3O cation. Craig beals shows how to draw the lewis structure for hydronium ion. Draw a Lewis structure for H3O Show all unshared electrons and the formal charges if any.
Here is the answer for the question which of the following species has a lewis structure similar to H3O. Assume that bonding follows the octet rule. From the above chart we can see that hydronium ion is a AX3E type molecule A central atom X bonded atom E lone pair on A.
So according to the VSEPR chart H3O has trigonal pyramid as its molecular shape and tetrahedral as its electron geometry. Solutions for problems in chapter 3. Note that the sign in the Lewis structure for H3O means that we have lost a valence electron.
Lewis Structure of NH 4 Q 5 4 x 1 1 8. Well finish by putting brackets around it like this here to show that its an ion and put a plus charge up here so everyone knows that its a positive ion a cation. These structures are written with a double-headed arrow between them indicating that none of the Lewis structures accurately describes the bonding but that the.
Draw a Lewis structure for H3O. For we first count valence electrons f. H3O is tetrahedral because when drawing the lewis structure there are a total of 8 electrons and so oxygen should have 3 bonds to hydrogens and then one lone pair which means there are four regions of electron density about the central atom which means the molecular geometry is tetrahedral but the shape is trigonal pyramidal.
Because according to Lewis concept of acid-base lone electron pair acceptors are Lewis acids. It is made up of one hydrogen one sulphur and four oxygen atoms. For we first count valence electrons the.
Draw the Lewis structure of H3O the hydronium ion. Charge of valence electrons nonbonding val bonding el. The Lewis structure of H 3 O is.
I also go over hybridization shape and bond angle. Thats the Lewis structure for H3O the hydronium ion. After that count the electrons and assign remaining lone pair electrons and the positive charge will be assigned over central atom.
Transcribed Image Textfrom this Question. 97 84 ratings FREE Expert Solution. There are 8 valence electrons for the H3O Lewis structure.
Which of the following is the correct Lewis Structure for the Hydronium Ion H3O. To be a Lewis acid the molecule or ion should possess a vacant orbital or a π-bond in which it can accept a lone electron pair. So in keeping with the VSEPR chart H3O has trigonal pyramid as its molecular form and tetrahedral as its electron geometry.
It is a conjugate acid of a water. Which of the following species has a lewis structure similar to H3O. Does h3o have resonance structures.
B and thanks for watching. In this video i will show the lewis structure ions hydronium ion h3o.

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

H3o Molecular Geometry Shape And Bond Angles Youtube

What Is The Shape Of H3o Ion Quora

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
Electron Dot Structure Of Hydronium Ion
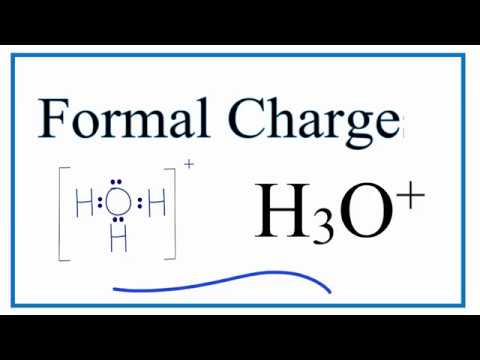
How To Calculate The Formal Charges For H3o Hydronium Ion Youtube

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

Chemistry Chemical Bonding 22 Of 35 Lewis Structures For Ions Hydronium Ion H3o Youtube

Electron Dot Structure Of Hydronium Ion Brainly In
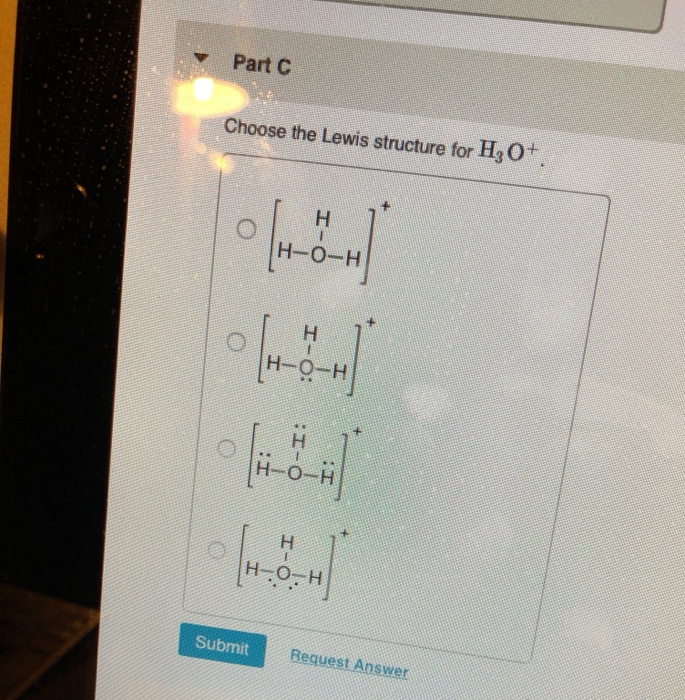
Part C Choose The Lewis Structure For H3o H O H Chegg Com

Draw An Electron Dot Diagram To Show The Structure Of Hydronium Ion Studyrankersonline
What Is The Structure Of H3o Quora

Draw The Lewis Structure For H3o And State Its Molecular Geometry Is It Polar Or Nonpolar Study Com

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
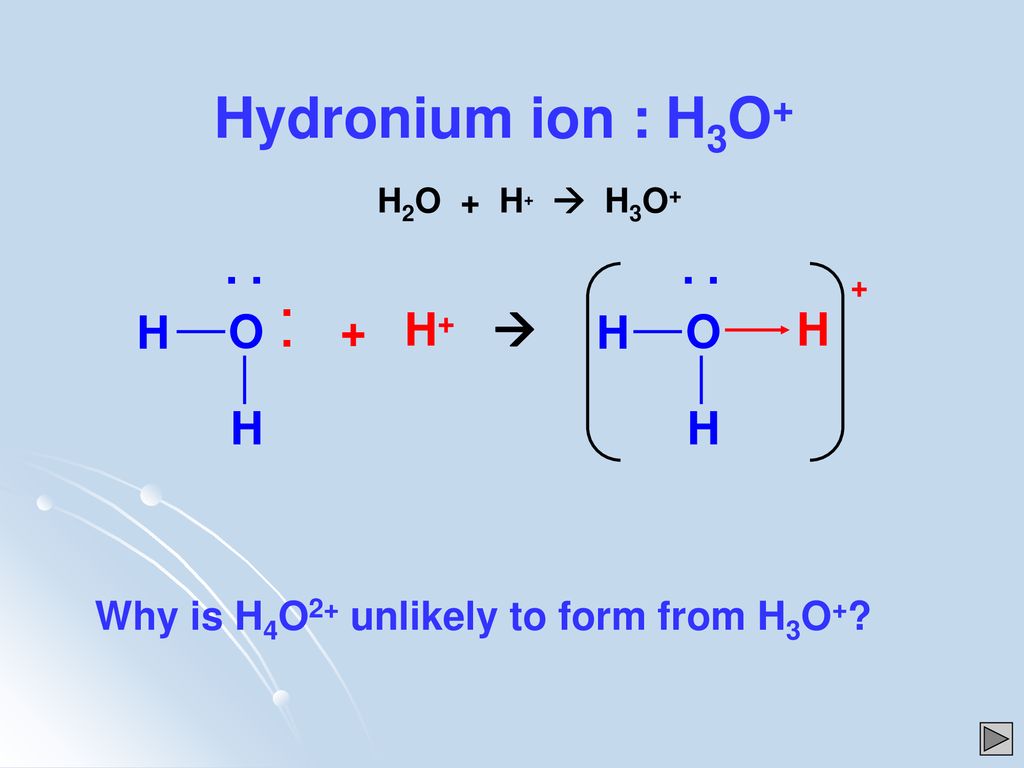
As Chemistry Coordinate Or Dative Covalent Bonding Nh4 Al2cl6 Co H3o Ppt Download
