Write The Lewis Structure Of Phosgene
A 1s 2 2s 2 2p 5 b 1s 2 2s 2 2p 6 3s 2. Complete the Lewis structures of these molecules by adding.

6 3 Molecular Shape Introductory Chemistry
1 3 2 4 1 3 14.
Write the lewis structure of phosgene. Write a balance chemical equation for the synthesis of phosgene 8 points. PROBLEM PageIndex7 The arrangement of atoms in several biologically important molecules is given here. If the molecule was ionic draw brackets around the Lewis structure and write the charge outside the bracket.
Write the Lewis structures for carbon tetrachloride and phosgene. Then write the Lewis symbol for the common ion formed from each atom. H 3 CCH 3.
Check me out. The Cl and O atoms bond to C c. Calculate the of electrons in π bonds pi bonds multiple bonds using formula 1 in the article entitled Lewis Structures and the Octet Rule.
Chemical Weapons Phosgene is a poisonous gas first used in chemical warfare during World War I. Write the Lewis Structures for. Where V 4 6 7 7 24.
2- Determine the group and the row of C O and Cl. Construct the Lewis structure model for the covalent compound phosgene COCl₂ using the following steps. Write Lewis structures for phosgene Cl 2 CO and formate ion HCOO.
Draw its Lewis structure. It has the formula mathrmCOCl_2mathrmC is the central atom. Write the Lewis structures for carbon tetrachloride and phosgene.
1 The total number of valence electrons in COCl₂ is _____. 1s 2 2s 2 2p 5 1s 2 2s 2 2p 6 3s 2. Both O atoms and the H atom are bonded to the C atom.
It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. The sum of the valence electrons is 5 from N 6 from. HCN is not ionic so no brackets are necessary and the above structure is the final Lewis structure.
5- Write the Lewis dot structure of COCl 2 and indicate the type of bonds between the atoms. Connect the atoms with single bonds. Then write the Lewis symbol for the common ion formed from each atom.
Where n in this case is 4 since COCl 2 consists of four atoms. To draw the Lewis structure for an odd-electron molecule like NO we follow the same five steps we would for other molecules but with a few minor changes. 1- Write the electron configuration of each element constituting phosgene.
Using formal charges determine which Lewis structure is the preferred one. Identify the atoms that correspond to each of the following electron configurations. 3- Write the Lewis electron dot symbol of C O and Cl.
It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. Draw three Lewis structures for the molecule that satisfy the octet rule for each atom. Identify the atoms that correspond to each of the following electron configurations.
Phosgene kills because it reacts with water in nasal passages in the lungs and on the skin to produce carbon dioxide and hydrogen chloride. It is no longer used for this purpose because of the formation of the toxic gas phosgene Cl 2 CO. 4- Identify the valence of each of the above elements.
Calculate the number of valence electrons. Write the Lewis structures for carbon tetrachloride and phosgene. Determine the total number of valence outer shell electrons.
Both O atoms and the H.

Construct The Lewis Structure Model For The Covalent Compound Phosgene Cocl2 Using The Following Steps 1 The Total Number Of Valence Electrons In Cocl2 Is 2 In This Compound Carbon Is

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Construct The Lewis Structure Model For The Covalent Compound Phosgene Cocl2 Using The Following Steps 1 The Total Number Of Valence Electrons In Cocl2 Is 2 In This Compound Carbon Is

Dot And Cross Structure For Cocl2 Phosgene Youtube
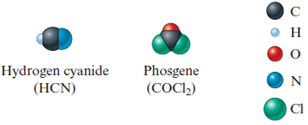
Solved The Space Filling Models Of Hydrogen Cyanide And Phosgene Chegg Com
Solved Phosgene Is The Acid Chloride Of Carbonic Acid Although Phosgene Was Used As A War Gas In World War I It Is Now Used As A Reagent For The Course

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Calculate The Formal Charge On Each Atom Of Carbonyl Chloride Brainly In
Solved Phosgene Is The Acid Chloride Of Carbonic Acid Although Phosgene Was Used As A War Gas In World War I It Is Now Used As A Reagent For The Course

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar
Question 2 1 Pts Calculate The Electronegativity Difference In The H H Bond Enter A Value Question Homeworklib
How To Calculate The Formal Charge Of Cocl2

Construct The Lewis Structure Model For The Covalent Compound Phosgene Cocl2 Using The Following Steps 1 The Total Number Of Valence Electrons In Cocl2 Is 2 In This Compound Carbon Is

S2f10 Disulfur Decafluoride Vintage Lewis Structure Etsy Matching Keychains Keychain Vintage
Solved Draw A Valid Lewis Structure For Phosgene Ccl 2 O Which Contains A Central Carbon Atom Phosgene Is An Extremely Toxic Gas Used As A Chemi Course Hero
4 4 Lewis Symbols And Structures General Chemistry 1 2
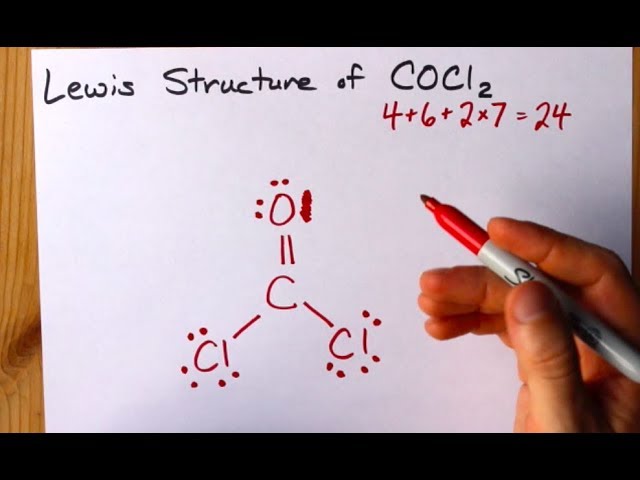
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube

Describe The Hybridization Of The Carbon Atom In The Poisonous Gas Phosgene Cl2co Study Com