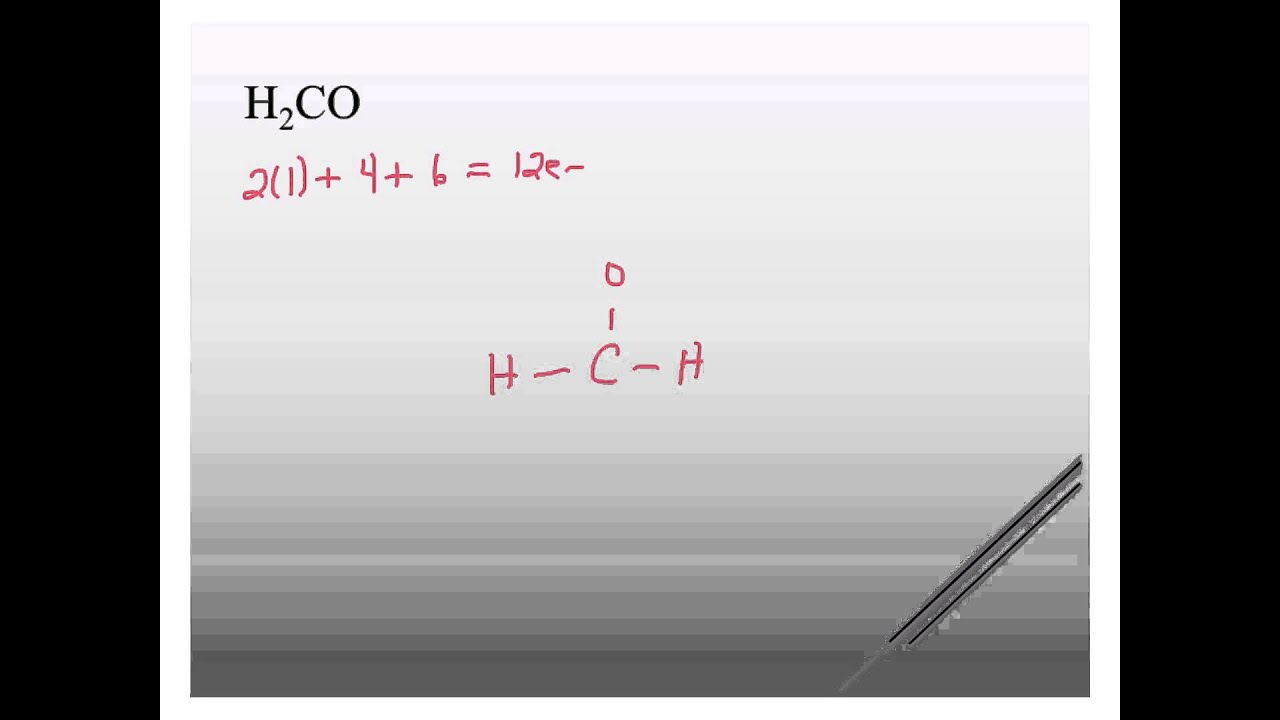
Structure De Lewis H2co
Alternatively a dot method can be used to draw the lewis structure. 605b Determine the formal charge on atoms in H2CO 317.

How To Draw The Lewis Dot Structure For Ch2o Formaldehyde Youtube
604c Draw the Lewis structure for CCL4 121.

Structure de lewis h2co. Space is limited so join now. Made with Explain Everything. Bonded pairs of electrons are represented by a single line.
Aldehydes are chemicals having the functional group -HCO- in their molecules and formaldehyde is the lowest member of this group with a single carbon atom. Want to see this answer and more. H2CO has 21 4 6 12 valence electrons.
H 2 CO is also called Formaldehyde. Instead the molecule is polar mainly due to the combination of its geometry and polarities of the chemical bonds taken individually. 605 Resonance Formal Charge 1627.
Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. The Lewis dot structure is an easy-to-use tool to help determine how a molecule in a covalently-bonded substance is put together. It has two H atoms one C atom and three O atoms.
We have previously discussed the Lewis structures of CO2 O3 SO2 SO3 and more. I also go over hybridization shape and bond angle. Drawing the Lewis Structure for H 3 O For the H3O Lewis structure we first count the valence electrons for the H3O molecule using the periodic table.
Draw the Lewis structure for H 2 CO. There are a total of 12 valence electrons in the H 2 CO Lewis structure. Embalming fluid and as a synthetic precursor to other organic.
It is an organic compound with the molecular formula of H2CO and is classified as an aldehyde. In the coming sections we will have a look at H2CO3 Lewis Structure Molecular Geometry Hybridization and MO Diagram. All Chemistry Practice Problems Lewis Dot Structures.
Draw the Lewis structure for H2CO. A Lewis structure is a drawing that shows all of a molecules valence electrons and all non-zero formal An example. What is the Lewis structure of H2CO.
604b Draw the Lewis structure for H2CO 200. 605a Determine the formal charg on atoms in NH4 224. That gives us a total of two electrons.
AX 3 has trigonal planarl shape. Use VSEPR table to find the shape. Use information from step 4 and 5 to draw the lewis structure.
This molecule has a plane of symmetry on which the all atoms lies on it. There are a total of 12 valence electrons in the H 2 CO Lewis structure. Lewis Structure of Carbonic Acid H2CO3 The formula of carbonic acid is H2CO3.
604d Draw the Lewis structure for NH3 116. ANumber of central atoms. For the above molecule VSEPR notation will be AX 3.
The polarity of the H2CO relies not only on electronegativities of carbon and oxygen solely. Once we know how many valence electrons there are in H3O we can distribute them around the central atom and attempt to fill the outer shells of each atom. Want to see the step-by-step answer.
Lewis dot structure of H 2 CO. The Lewis Dot Structure for H 2 H 2 CO formaldehyde is used as a tissue preservative eg. XNumber of surrounding atoms.
Carbon goes in the centreMake sure carbon and oxygen get 8 electrons to fulfil octet rule. Use lewis structure guidelines to draw the lewis structure of H 2 CO. Check out a sample QA here.
Neutral Compounds Practice Problems. Apply VSEPR notation A X E. Calculate the total valence electrons in the molecule.
Para un anión poliatómico se le añade un e- más por cada c. 604a Draw the Lewis structure for H2O 113. E Number of lone pairs on central atom.
Determine the formal charge on atoms in H2CO. Drawing the Lewis Structure for H2CO. Draw the Lewis structure of formaldehyde H₂CO.
Experts are waiting 247 to provide step-by-step solutions in as fast as 30 minutes. Neutral Compounds Concept Videos. A Lewis structure is a drawing that shows all of a molecules valence electrons and all non-zero formal An example.
Formaldehyde Methanal H2CO is a trigonal planar molecule AX3 geometry 120 degree bond angle. H2CO Lewis Structure Molecular Geometry Hybridization and MO Diagram. H 2 CO is the simpliest example of the organic functional group called the Aldehydes.
Today we are going to learn about the Lewis structure of H2O molecule along with its molecular geometry and shape. Learn this topic by watching Lewis Dot Structures. I quickly take you through how to draw the Lewis Structure of water H2O.
Carbon C is the least electronegative atom and goes at the center of the H 2 CO Lewis structure. To complete the Lewis structure just add lone pairs of electrons to satisfy the octet rule for the atoms that have. Here is its Lewis Structure and VSEPR shapeCheck me out.

Chemical Bonding Learning Objectives To Understand What Covalent Bonding Is To Predict When Covalent Bonding Will Occur To Use Chemical Formulas To Ppt Download

Chemistry Molecular Structure 12 Of 45 Basic Shapes Predict The Shape Of H2co Youtube

6 02 Draw The Lewis Structure For H2co Youtube
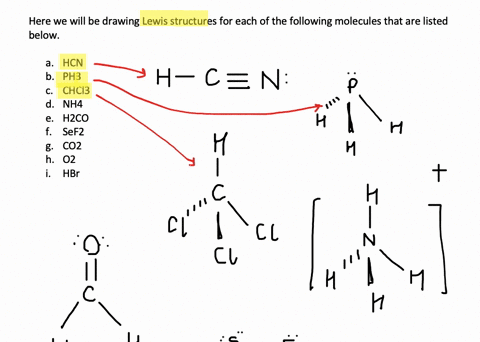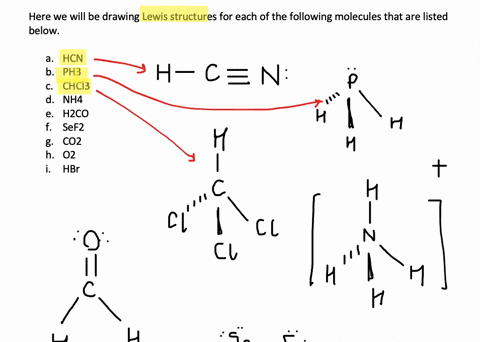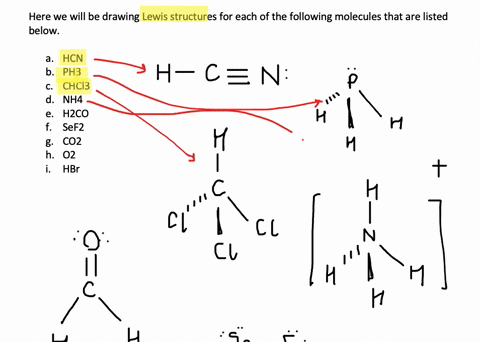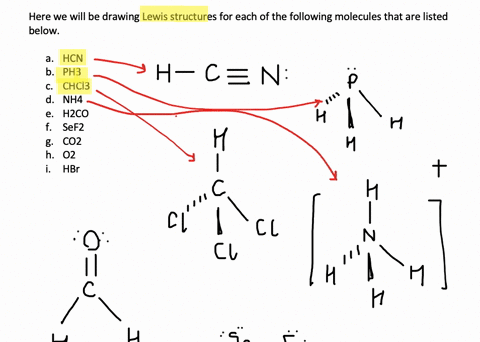
Solved Write Lewis Structures For The Following

How To Draw Lewis Structure For Hclo4 Drawing Easy
What Is The Lewis Structure Of Ch2o Quora

Lewis Structure Vsepr For H2co Youtube
H2co Lewis Structure How To Draw The Lewis Structure For H2co دیدئو Dideo
Solved Write Lewis Structures For The Following A O2 B H2co C Asf3 D Clno E Sicl4 F H3o G Nh4 H Bf4 I Hcch J Clcn K C2 2 Course Hero

Lewis Structure Of H2co Biochemhelp
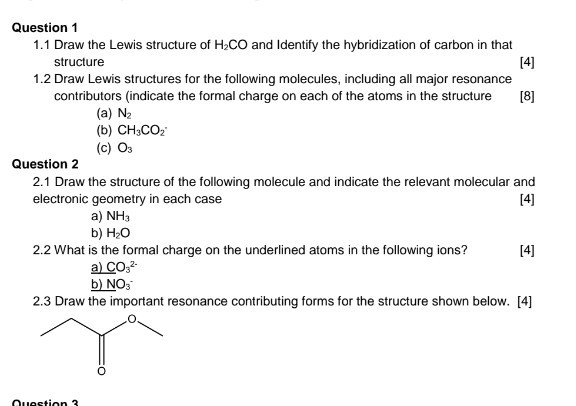
Question 1 1 1 Draw The Lewis Structure Of H2co And Chegg Com

H2co Lewis Structure How To Draw The Electron Dot Structure For H2co
H2co Lewis Structure How To Draw The Lewis Structure For H2co دیدئو Dideo
Chem Filling In The Valence Electrons Of An Electron Dot Structure Lewis Structure Scientific Tutor

Drawing Lewis Structures Testing

How Do I Use The Lewis Structure Drawing Tool 101edu