Draw The Lewis Structure For Co2
Here are the steps that I follow when drawing a Lewis structure. Carbon is the least electronegative that means it is going to go to the center.
For the CO 2 Lewis structure there are.

Draw the lewis structure for co2. That will normally be the least electronegative atom C. That would mean that you would have a total of eight dots around the carbon thereby filling its octet. To draw the Lewis Dot structure for CO2 we have to find out the CO2 valence electrons firstWe express valence electrons as dots in CO2 lewis dot structure.
CO2 4 62 16. If the species contains oxygen do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. Draw The Lewis Structure For Co2 Lewis dot structure of CO2 Draw the lewis dot structure of co2 3 Chemistry CO2 Lewis dot structure diagram YouTube Calculating CO2 Formal Charges.
Oxygen has six valence electrons. To get the valence electrons of carbonwe need to look at the electronic configuration of carbon. A step-by-step explanation of how to draw the COCarbon Monoxide Lewis Dot DiagramFor the CO structure use the periodic table to find the total number of v.
Clectrons 2 Draw a Lewis structure for CO2 Do not include overall ion charges or fornal charges in your drawing. This chemistry video tutorial explains how to draw the lewis structure of CO2 also known as Carbon Dioxide. Decide which is the central atom in the structure.
Lewis structure for CO2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. C 61s²2s²2p² The highest value of principal quantum number here is n2.
Draw a trial structure by putting electron pairs around every atom until each gets an octet. The Lewis dot structure is drawn with letters that represent the atoms of the element and then a number of dots or dashes surrounding these letters. In the CO 2 Lewis structure carbon is the least electronegative element.
Lets go over the Lewis structure and find out how to interpret this representation of carbon dioxide. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. Draw The Lewis Structure For Co2 C Is The Central Atom Bonding in the CH2O Molecule YouTube 09 polarity 2016 ICl4 Lewis Structure How to Draw the Lewis Structure Chapter 8 docx Chapter 8 8 Draw the Lewis structures.
CO 2 is a clear heavier-than-air gas. The carbon dioxide chemical formula is CO2. Drawing lines represent the bonds formed in the.
These valence electrons are represented by drawing dots around the individual atoms hence the Lewis dot structure. Lets draw the structure. This is the Lewis Dot Structure for CO2.
It also discusses the bond angle molecular geom. Here in this post we described step by. Draw A Lewis Structure For Co2 lewis co2 dot structure dioxide carbon diagram electron pairs resonance electrons structures bonds elements many covalent lone valence oxygen ionic lewis co2 structure carbon dioxide draw centre oxygens either put going then side go dot diagram dioxide lewis molecular cross co2 structure structures silicon carbon draw chemistry co2 dot lewis diagram structure.
Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons. Dots can be used to represent the shared electrons within the bonds of the atoms but dashes can be used. Therefore it is put in the center of the dot structure.
Count the valence electrons in your trial structure. To know the lewis structure of CO2 one should first understand what precisely the Lewis structure is. Drawing CO2 Lewis Structure is very easy to by using the following method.
In the Earths atomsphere it is considered a greenhouse gas. How To Read A Lewis Dot Structure. From the periodic table Carbon has four valence electrons.
You follow a sequence of steps. You could alternatively also draw the structure by including two dots for every bond. Put the Carbon at the center and then Oxygen on either side of that.
1 What is the total number of valence electrons in the Lewis structure of CO2. The octets of both of the oxygen atoms are also satisfied since the oxygens have a total of eight electrons around them thereby filling the valence shell. In order to complete the octets for all of the atoms in the structure you will need to form two double bonds.

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization
Co2 Lewis Structure Molecular Geometry And Hybridization
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

Makethebrainhappy The Lewis Dot Structure For Co2

Co2 Lewis Structure Carbon Dioxide Youtube
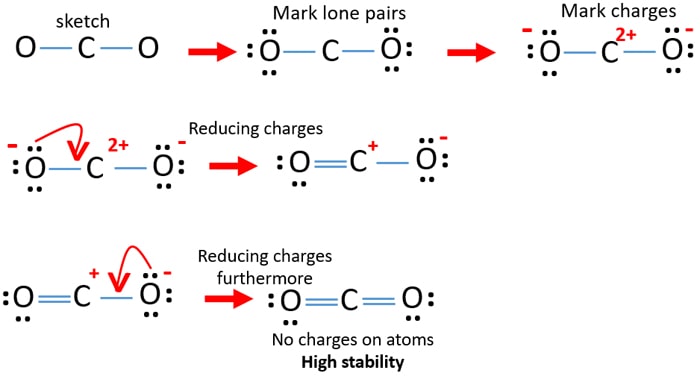
Co2 Carbon Dioxide Lewis Structure And Shape

Co2 Lewis Structure Easy Hard Science
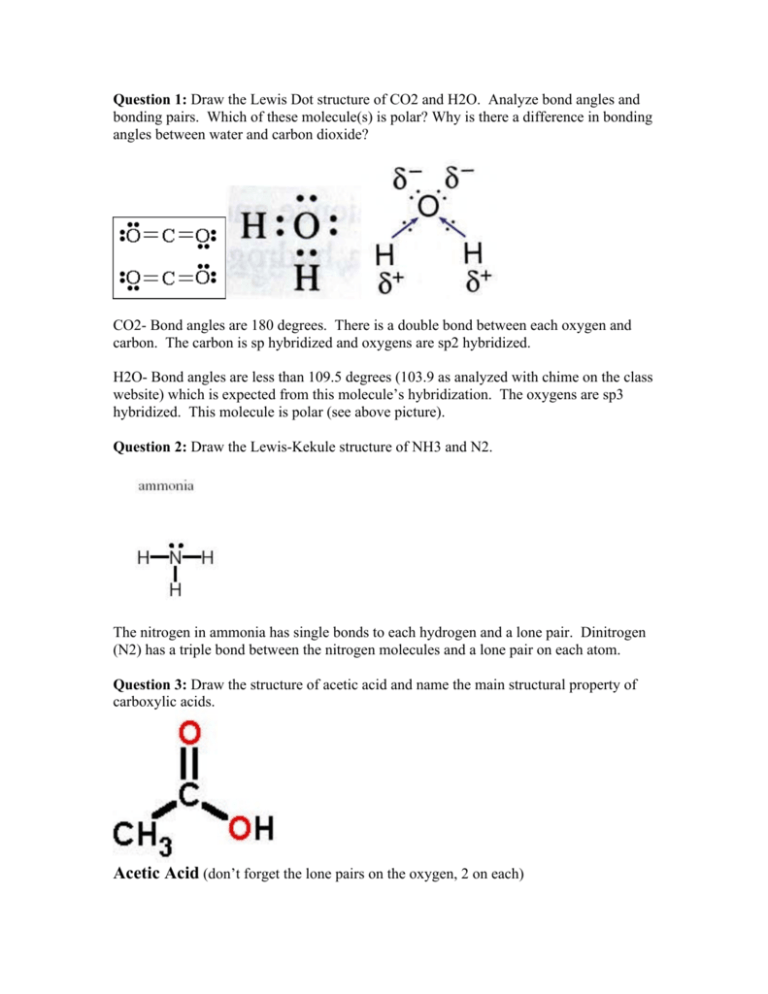
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

Co2 Lewis Structure And Molecular Geometry What S Insight
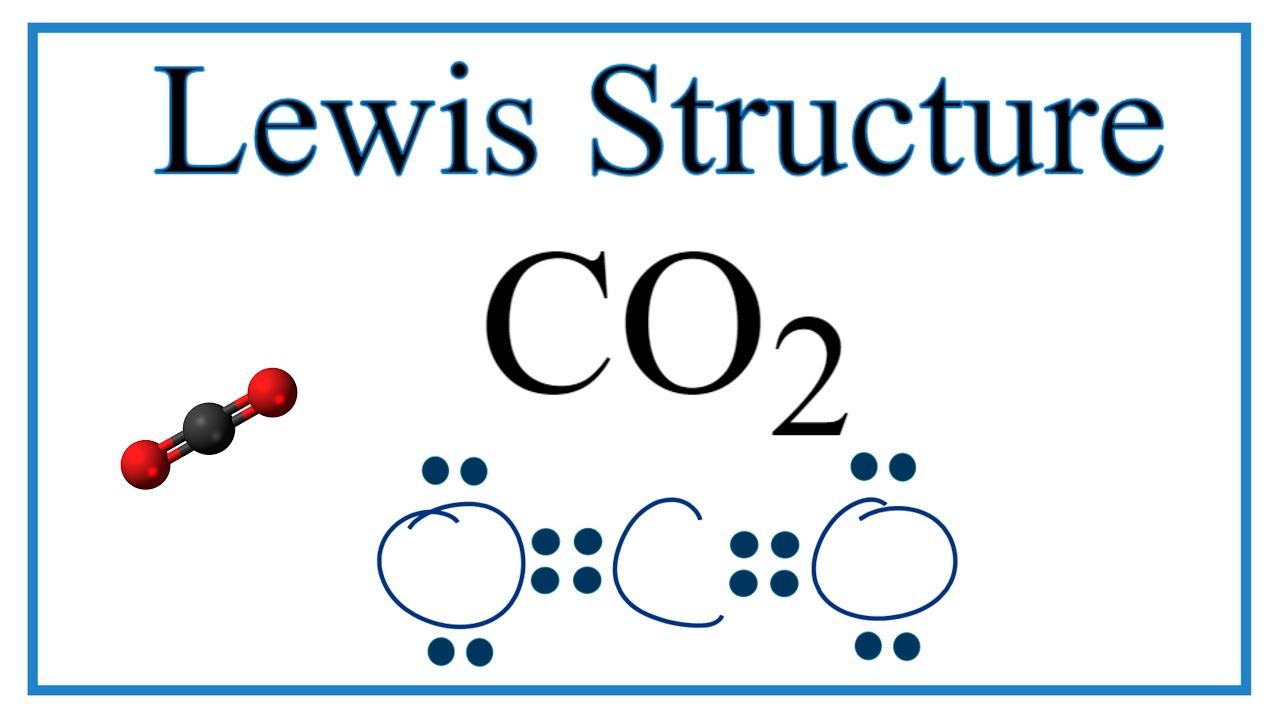
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube

Lewis Structure For Co Carbon Monoxide Youtube

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube

Co2 Lewis Structure Easy Hard Science

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Co2 Lewis Structure Carbon Dioxide Youtube

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
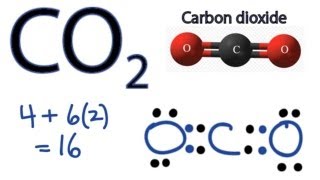
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

What Is The Lewis Structure Of Co2 Clutch Prep
