What Is The Electron Geometry Of Clf5
Site Report a Problem Comments Suggestions Stoichiometry. You will need to place the remaining two valence electrons on.

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
The group 5A elements arent as recognizable as other elements on the.
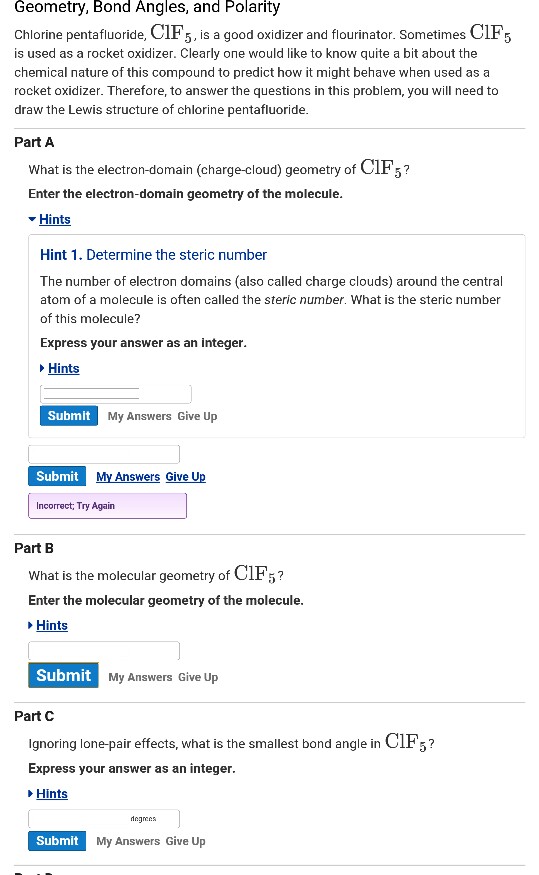
What is the electron geometry of clf5. It appears to be asymmetrical and is polar. Is the moleculepolar or nonpolar. Thus its electron-domain geometry is octahedral.
In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms. There are 5 Cl-F bonds in this structure. ClF5 has 42 electrons to account for.
Homework Help Resource Prentice Hall. Become a member and. What is the moleular geometry of ClF5.
For homework help in math chemistry and physics. Why can Xe have an expanded octet. Answer the following question for ClF5.
What is the electron-domain charge-cloud. The magnitude of the electric force between these two charges is 395 x 106 N. The molecules consists of five single bonds each connecting a fluorine to the central Cl and one.
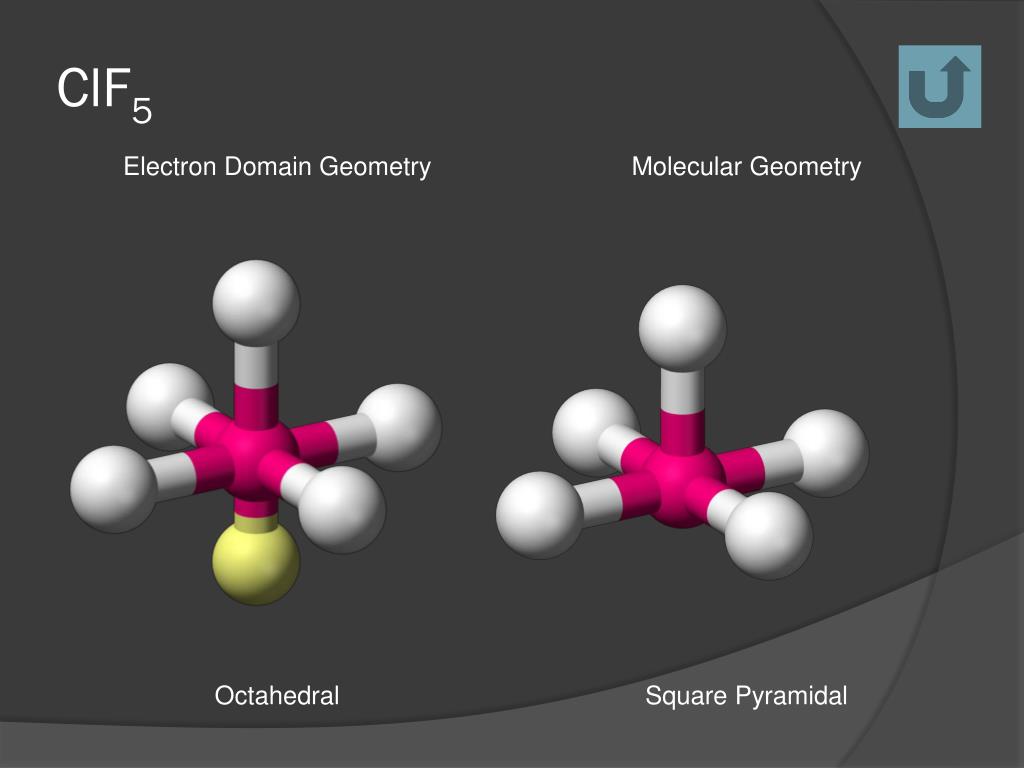
Contrast SF6 with ClF5. What is the molecular geometry of ClF5. This corresponds to AX 6 or octahedral.
Study Guide Test Prep Principles of Physical Science. Chlorine Pentafluoride on Wikipedia. The molecular geometry of ClF 5 is square pyramidal.
There are 5 atoms and 1 lone pair or a total of 6 electrons around the central atom Cl. It is a eqsp_3d2 eq and the electron-domain geometry of Chlorine pentafluoride is octahedral. ILTS Science - Physics 116.
High geometries sp3d2 hybrid. Answer and Explanation. For ClF5 Cl goes in the center since it is the least electronegative.
A Cl-F bond is a dipole since F is more electronegative. Equivalent forces rather than just bonds and atoms has 42 electrons in PCl5. Molecular Geometry Polarity Tutorial.
The electron domain charge cloud geometry of clf5 is a molecule that has five fluorine atoms. This is called an expanded. Beside above is ClF5 polar or nonpolar.
In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms. As per the VSEPR theory valence shell electron. Answer the following question for ClF5.
Learn vocabulary terms and more with flashcards games and other study tools. The electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. Is the moleculepolar or nonpolar.
As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal. Its also known as pentafluoropentane or C 5 F 12. Predict the central atom of the molecule.
Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. Back to Molecular Geometries Polarity Tutorial. The electron geometry of ClF5 is AX6 or octahedral.
This compound belongs to the family of hydrocarbons which are compounds consisting only of hydrogen and carbon. Two electrons are left on Cl as a lone pair. Start studying chem exam 3 - ch 7 Copied from somone els.
Electrons can be termed the domainof the electron domain and molecular clf5 lewis structure molecular geometry will be square pyramidal and is to. Study Guide Test Prep DSST Principles of Physical Science. The geometry is square pyramidal and is due to 6 electrons pairs around the central chlorine atom one of which is nonbonding.
See full answer below. Additionally what is the Lewis structure for ClF5. Help and Review AP Environmental Science.
Therefore IF 5 is polar. What is the electron geometry of ClF5. The main difference between electron geometry and molecular geometry is that electron geometry is found by taking both lone electron pairs and bonds in a molecule whereas molecular geometry is found using only the bonds present in the molecule.
As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal. Does ClF5 have a dipole moment. Since there are six electron pairs on the central atom then VSEPR theory tells us that the electron pair geometry is octahedral and with 5 bonding pairs and 1 lone pair the molecular geometry is.
Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. Because of this symmetrical geometry CCl 4 is non-polar. Therefore this molecule is polar.
30 more are in lone pairs on F atoms. 5 Lewis structure of Cl 2 O 2 based on the central Chlorine atom polar â. What is the moleular geometry of ClF5.
Xenon now has twelve electrons instead of the octet. Test Prep Practice UExcel Earth Science. Certificate Program AP Environmental Science.
The molecular geometry of ClF 5 is square pyramidal with asymmetric charge distribution around the central atom. ClF5 however is out of balance and therefore does have a dipole. Test Practice and Study Guide NY Regents Exam - Living Environment.
A The molecule is polar and has polar bonds. The electron geometry of ClF 5 is AX 6 or octahedral. Both of these have an sp3d2 hybrid about the central atom.
A thundercloud has an electric charge of 432 C near the top of the cloud and -387 C near the bottom of the cloud. That accounts for 40 electrons. What is the electron geometry of ClF5 ClF 5.
What is the electron geometry of ClF5. 10 of them are in bonding pairs of electrons.
2 10 16 Today I Will Determine The Shapes Of Small Molecules Ppt Download
Chlorine Pentafluoride Clf5 Lewis Structure Lewis Structure For Brf5 Molecular Geometry Bond Angle Hybridization Polar Or Nonpolar Just Another
Chapter 8 Covalent Compounds Bonding Theories And Molecular Structure

What Is The Molecular Geometry Of Clf5 Enter The Molecular Clutch Prep
What Is The Shape Of Clf5 Using Vsepr Theory Quora

What Is The Molecular Geometry Of Clf5 Enter The Molecular Clutch Prep

Vsepr The Familiar Vsepr Valence Shell Electron Pair Repulsion Approach To Molecular Structure Was Developed By Ronald Gillespie The Basic Idea Is Ppt Download
What Is The Shape Of Clf5 Using Vsepr Theory Quora

Vsepr Molecular Geometry Example 2 Clf5 Youtube

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Molecular Structure And Polarity Ns 104 General Chemistry 1 Svc Openstax Cnx

Ppt Molecular Geometry Powerpoint Presentation Free Download Id 3887508

Download Chlorine Pentafluoride Clf5 Lewis Dot Structure 3gp Mp4 Codedwap
Geometry Bond Angles And Polarity Chlorine Chegg Com
Clf5lewis Structure Iae News Site
Is Clf5 Polar Or Non Polar Quora

What Is The Molecular Geometry Of Clf5 Enter The Molecular Clutch Prep