Is Sif4 A Lewis Base
Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. CF4 can not act as Lewis acid.
Part B For The Following Reaction Identify The Lewis Chegg Com
Which are Lewis acids and which are Lewis basesa Mg2 b OH c SiF4 d BeCl2 Q.

Is sif4 a lewis base. Lewis Bases are Nucleophilic meaning that they attack a positive charge with their lone pair. CF4 can not act as Lewis acid. What is the difficulty of this problem.
Anything that is e- deficient is a Lewis acid. They utilize the highest occupied molecular orbital or HOMO Figure 2. Is sicl4 polar or nonpolar.
Hence they can not act as Lewis Acid eg. Fastest Reliable Cheapest Game Keys and Digital Services at DamnModz. The correct option is.
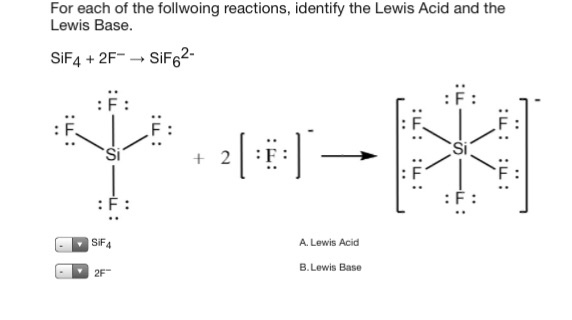
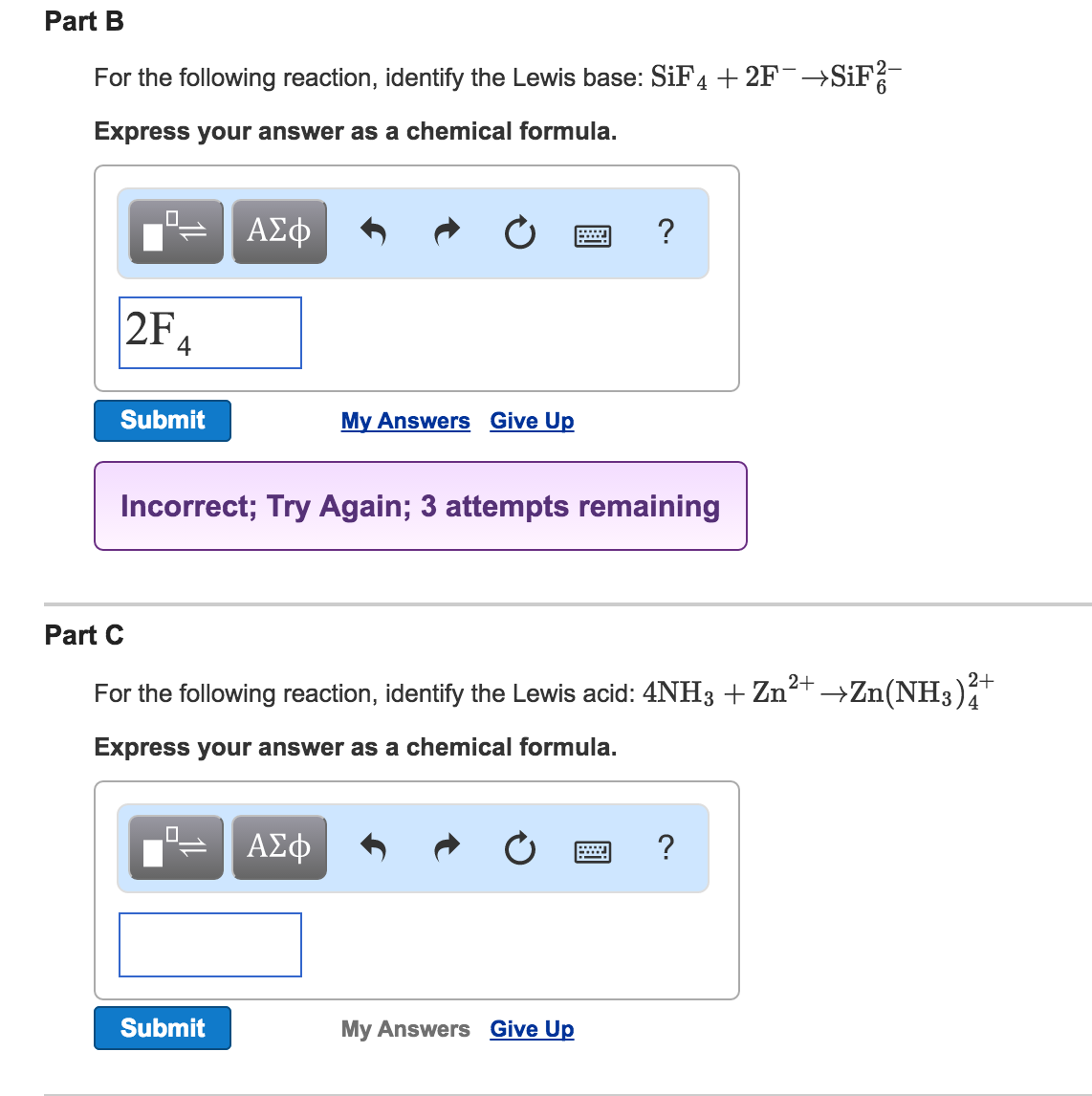
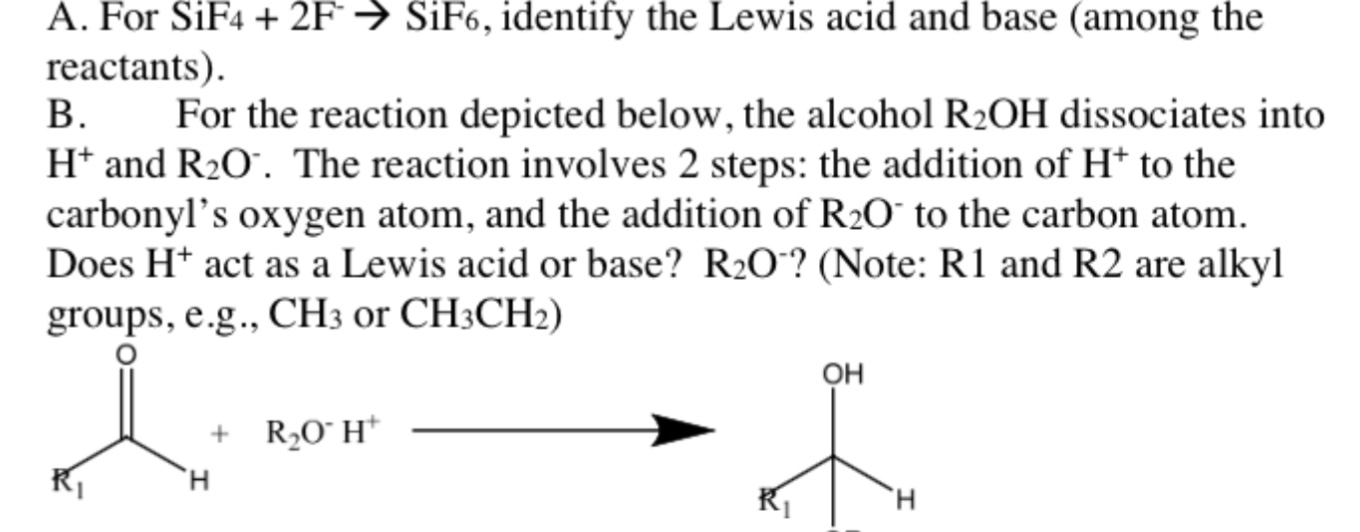
Lewis acid can accept a pair of electron from Lewis base. SiF4 2F- SiF62- SF4 F- SF5- Its taking that negative charge electrons from the F-Be careful not to confuses these with the Bronsted-Lowery acids. In many cases the Lewis acid can bind two Lewis base a popular example being the formation of the hexafluorosilicate.
Its also a pi acceptor Lewis acid. Heres how SiF4 acts like a Lewis acid. Search More info Main menu.
Indeed the required interaction would be the Lewis bases lone pair with sigma_ceC-F an orbital which is already well hidden by the three other. Is AlCl3 an acid or base. Lewis acid can accept a pair of electron from Lewis base.
Which are Lewis acids and which are Lewis basesa Na b NH3 c CN d BF3. Aluminum chloride AlCl3 is a Lewis acid since an open valence shell is found in the aluminium atom. An atom ion or molecule with a lone-pair of electrons can thus be a Lewis base.
SiF4 2 F SiF62. S F 4 can act as Lewis acid. Ralph Pearson classified all Lewis acids and bases as hard and soft acids and bases.
Find more Chemistry widgets in. Lewis Bases donate an electron pair. Please Choose Either A Or B.
Hard acids have small acceptor atoms of low polarisability and. Our tutors rated the difficulty of Is SiF4 a lewis base or lewis acid. Let us help you simplify your studying.
C The overall charge of the molecular species. Cu2 accepts electron pairs in order to make complexes. Soft acids have large acceptor atoms of low positive charge high polarisability and low electronegativity.
Br2 and Br are soft Lewis acids and Br- has properties in between soft bases and hard bases. Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg. F- to give SiF6 2- ion.
Is SiF4 A Lewis Base Or Lewis AcidIs BeCl2 A Lewis Base Or Lewis Acid. The weakly acidic species silicon tetrachloride SiCl4 can be activated by binding of a strongly Lewis basic chiral phosphoramide leading to in situ formation of a chiral Lewis acid. An atom ion or molecule with an incomplete electron octet may be known as Lewis.
Hence they can not act as Lewis Acid eg. Under the Lewis definition hydroxide acts as the Lewis base donating its electron pair to H. SARL K FOOD COMPANY.
The fluorides BF3 AIF3 SIF4 and PF5 are Lewis acids. Hence Br2 is considered to be a soft Lewis acid. Thus in this version of the neutralization reaction what interests us is not the salt that forms but the covalent bond that forms between OH and H to form water.
H BF3 AlCl3 SO2 SO3 CO2 NO2 etc. None of the above is a Lewis acid. Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg.
B C2H4 ExplanationIn BF3 and FeCl3 molecules the central atoms have incomplete octet and in SiF4 the central atom has empty d-orbitals. A Lewis base is a chemical compound that can donate a pair of electrons to a suitable electron-pair acceptor Lewis acid to form a Lewis adduct. Is C2H4 a Lewis acid or base.
SF4 has a lone pair on the S. F- to give SiF6 2- ion. On the other hand such process is not possible for compounds formed by 2nd period elements.
Is NO2 a Lewis acid or base. Or if you need more Lewis Acid and Base practice you can also practice Lewis Acid and Base practice problems. Similarly popular cases are the aluminum trihalides that are visible widely as Lewis acids.
Each of the following anions can give up their electrons to an acid eg OH- CN- CH_3COO- NH_3 H_2O CO. Reason The compound which contains vacant d-orbitals can act as Lewis acid. Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base.
On the other hand such process is not possible for compounds formed by 2nd period elements. Hence according to Lewis concept these are Lewis acids. Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base.

Is Sif4 A Polar Or A Non Polar Molecule Quora

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora
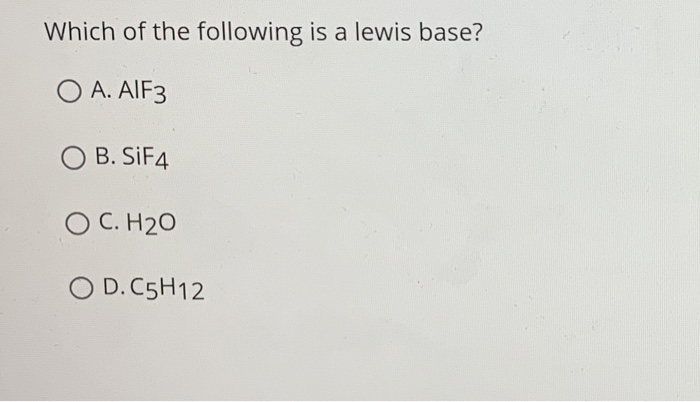
Which Of The Following Is A Lewis Base O A Alf3 O Chegg Com

Lewis Acid And Base Concept For Each Of The Following Chegg Com

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F

Is Sif4 A Lewis Base Or Lewis Acid
A For Sif4 2f Sif6 Identify The Lewis Acid And Chegg Com

Which Are Of The Following Are Lewis Acids Cheek Chegg Com

Is Sif4 A Lewis Base Or Lewis Acid
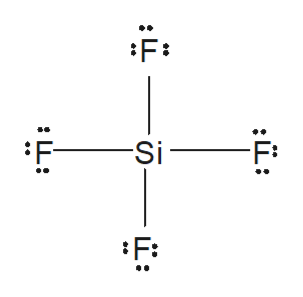
Answered From The Lewis Diagram Of Sif4 Bartleby

Solution Which Of The Following Is A Lew Chemistry
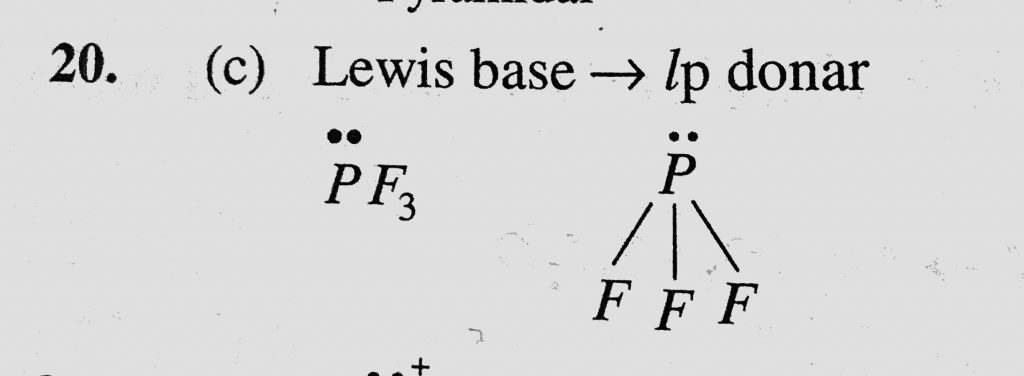
Which Of The Following Fluoro Compounds Is Most Likely To Behave As A Lewis Base A Sif4 B Bf3 C Pf3 D Cf4 Sahay Lms
Chem Molecular Shape Molecular Geometry Scientific Tutor
For Each Of The Following Reactions Identify The Chegg Com
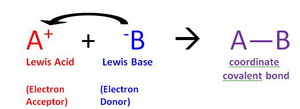
Why Does Sif4 Act As A Lewis Acid Example
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero
What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora