C2h2 Lewis Structure Bonds
In reality the molecular shape of ethene is not linear. O 2 double bonds and 2 lone pairs.

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
Number of lone pairs on an interior atom.
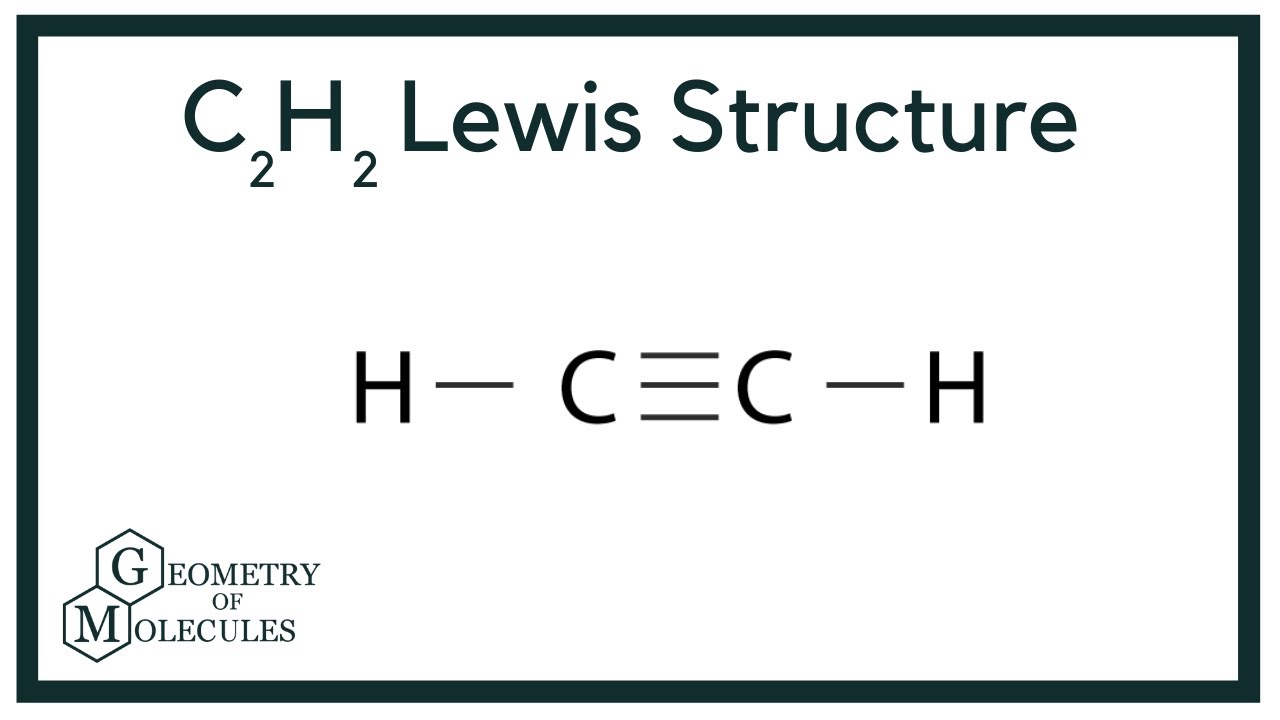
C2h2 lewis structure bonds. 114 What are Lewis structures. In Lewis structure of C 2 H 2 the octets of all the atoms are complete and there are no lone pairs of electrons in the molecule. Note the Hydrogen atoms H should not have lone pair.
Ethyne acetylene C2H2 HCCH Lewis structure using dots to represent bonding electrons. The Lewis structure of C 2 H 2 is. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for.
Draw the molecule by placing atoms on the grid and. Hence C2H4 is an alkene. In PCI Lewis structure each chlorine atown is joint with centere phosphorus atom through a single bond Also is a lone pair on phosphorus atom.
White the lewis structure of the following compounds. Hydrogen has one bond and no lone pairs. C2h2 Lewis Structure Triple Bond.
Drawing the Lewis Structure for C2H2 Ethyne or Acetylene For C2H2 you have a total of 10 valence electrons to work with. Acetylene Chemical Formula Lewis Structure Molecule. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds.
Commonly called acetylene has o 2 single bonds 1 triple bond and 1 lone pair. Number of bonding groupspairs around an interior atom. When we draw the Lewis Structure of C2H4 we find a linear 2-D representation.
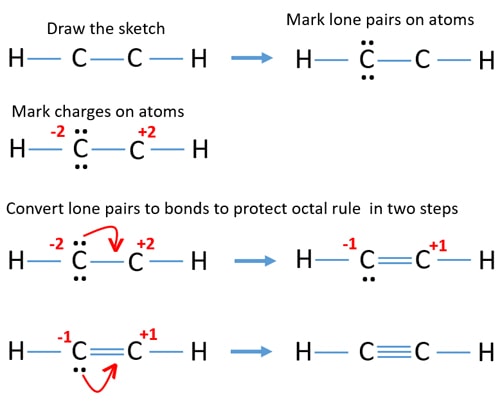
The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C2H2. As we know that carbon has 4 valence electrons and hydrogen has 1 valence electron. Lewis structure of HCN Uncharged nitrogen has three bonds and one lone pair.
Question 7 1 pts The best Lewis structure for ethyne C2H2. The Lewis structure of C 2 H 2 helps us understand the geometry of the molecule. C 2 H 2 Acetylene Ethyne Lewis Structure C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms.
Chemistry questions and answers. Total number of electron groups around each identical interior carbon atom. O 2 single bonds 1 triple bond and no lone pairs.
Lewis structure using lines to represent pairs of bonding electrons. In C2H2 Lewis structurethree double bonds exist between the two carbon atomsBesideseach carbon has one hydrogen atom using single bondIn C2H2 Lewis Dot structurethere are totally ten valence electrons. In the Lewis structure for acetylene the three lines between the carbon atoms represent three bonds between the carbon atoms.
Put carbon in the center and arrange hydrogen and nitrogen atoms on the sides. A total of six valence electrons is used to form a triple bond between both the Carbon atoms. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
In drawing the lewis structure for c2h2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. C2h2 Lewis Structure Bonds C2H2 Lewis Structure Tutorial How to Draw the Lewis Draw the molecules Include all lone pairs of electrons Tang 08 hybridization Production of Materials Ethylenes Bonding and Products. A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles.
Phosphate trichloride PC contain three chlorine atoms and one phosphorus atoms. Since there are two bonds forming here we will have a double bond structure. The number of sigma and pi-bonds are 6 and 2 respectively.
Why is C2H2 a triple bond. Here we have got the most suitable and appropriate Lewis Structure Sketch of ethylene. O 2 single bonds 1 double bond and 2 lone pairs.
Carbon has 4 valence electrons hydrogen has 1 valence electrons oxygen has 6 valence electrons total valence electrons 1 carbon. According to Lewis-dot structure there are 16 number of bonding electrons and 0 number of non-bonding electrons. There are no lone pairs on carbon or hydrogen atoms.
All the atoms here lie in the same plane and there is no. Each carbon atom has 4 valence electrons and each hydrogen has 2 valence electrons. Arrange electrons until both carbon and nitrogen get a triple bond giving an octet and hydrogen has 2.
It has 3 σ-bond and 2 π bond. Ethane C2H6 Ethene C2H4 And Ethyne C2H2 Ea.

Draw The Lewis Structure For Acetylene C2 Clutch Prep

C2h2 Lewis Structure Ethyne Or Acetylene Youtube

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist

C2h2 Lewis Dot Structure Geometry Youtube

Lewis Structure For C2h2 Ethyne
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Is C2h2 Polar Or Nonpolar All About C2h2 Polarity
C2h2 Molecular Geometry Shape And Bond Angles See Description For Note دیدئو Dideo

C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
C2h2 Acetylene Ethyne Lewis Structure
Lewis Structure For C2h2 Ethyne

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

Molecular Geometry Of Acetylene Chemistry Stack Exchange

What Is The Lewis Structure Of C2h2 Study Com
C2h2 Lewis Structure Tutorial How To Draw The Lewis Structure For Ethyne Or Acetylene دیدئو Dideo

C2h2 Acetylene Ethyne Lewis Structure
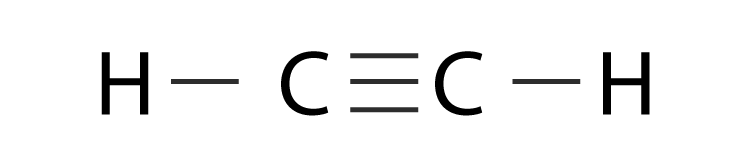
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne