C2h4 Lewis Structure Shape
Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons. Finding the valence electrons for each atom.

Chemistry Molecular Structure 15 Of 45 Basic Shapes Predict The Shape Of C2h4 Youtube
From the structure determine the shape of the molecule based on the carbon atom.

C2h4 lewis structure shape. Using steric numbers or the A-X-N method we can determine the molecular structure for C 2 H 4. Ethylene C2H4 has the Lewis Structure. This means that the.
Ethane Hybridization Molecular Geometry and shape. In a double bond two pairs of valence electrons are shared for a total of four valence electrons. This is a 2-D representation and it helps us to understand more about the properties of the compound.
There are two triangles overlapping each other as we can see in the diagram. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. Draw the electron-dot structure for C2H4.
According to the VSEPR chart the shape of the ethene molecule is trigonal planar. See the title structure below Flat but I cant see anything from the angle. By signing up youll get thousands of.
It is a chemical formula for Ethylene or Ethene. As per the Ethene Lewis dot structure Four CH sigma bonds are present and one CC double bond1 sigma 1 pie bond. The molecular geometry of C2H4 is a trigonal planar with respect to carbon left and right.
C2H6 lewis structure. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. The molecular shape is expected to be a flat triangle around each carbon atom.
Look at the structure for each molecule and determine if your lewis structure and shapes were correct. For C 2 H 4 you have a total of 12 total valence electrons. Drawing the Lewis Structure for C 2 H 4.
This video teaches you how to draw the Lewis Structures and themoleculargeometry for ethylene C2H4. Chemistry questions and answers. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
They say both shape and angle here. Ethane is an organic compound with a chemical formula of C2H6. C2h4 Molecular Geometry What is the shape of the molecule and the binding angle of C2H4.
Hence total shared pairs of electrons in the Lewis dot structure of C2H4 is 12. Substance Lewis Structure Valence Electrons Zones e-Pair Molecular Geometry Shape C2H4 PC13 C2H6 C2H2 NH4 Xe0F4 C2H2Cl2 SO2 SOCl2 COCI2. Draw the Lewis structure of C2H4.
Drawing the Lewis dot structure for C2H4 ethene and answer the questions below. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. C2H4 Molecular Geometry As it can be seen from the Lewis structure above each Carbon atom is bonded to two Hydrogen atoms.
Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure. Molecular geometry This is ethane an alkyne double H to H with 2 carbon atoms which means that the relationship between the carbon atoms is double. The molecular shape is predicted to be trigonal planar around each carbon atom.
Many people are confused that C2H4 is tetrahedral but its not tetrahedral C2H4 has a trigonal planar shape. Answer to Substance Lewis Structure Valence Electrons Zones. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair.
Trigonal pyramidal Tetrahedral O Linear Trigonal planar. It means four CH bond has 8 shared pairs of electrons and CC bond has 4 shared pairs of electrons. This is composed of a σ.
How many shared pairs of electrons are in the lewis dot structure of C2H4. Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence.
Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. Is it polar or nonpolar. These Hydrogen atoms repel each other on the same plane according to the VSEPR theory.
What is its molecular shape. Lets move step-by-step and see how the Lewis Structure of C2H5OH can be made.
C2h4 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Ch 10 Vsepr Practice Problems 3 Flashcards Quizlet

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
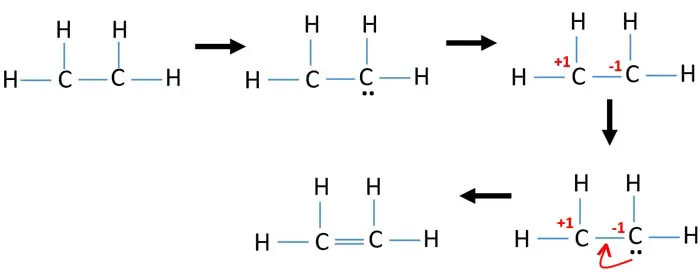
Ethene C2h4 Lewis Structure Hybridization
How Is C2h4 Planar While C2h6 Is Non Planar Quora

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Molecular Geometry Shape And Bond Angles Youtube

Wn C2h3f Lewis Structure Polar Or Nonpolar Bond Angle Molecular Geometry Hybridization
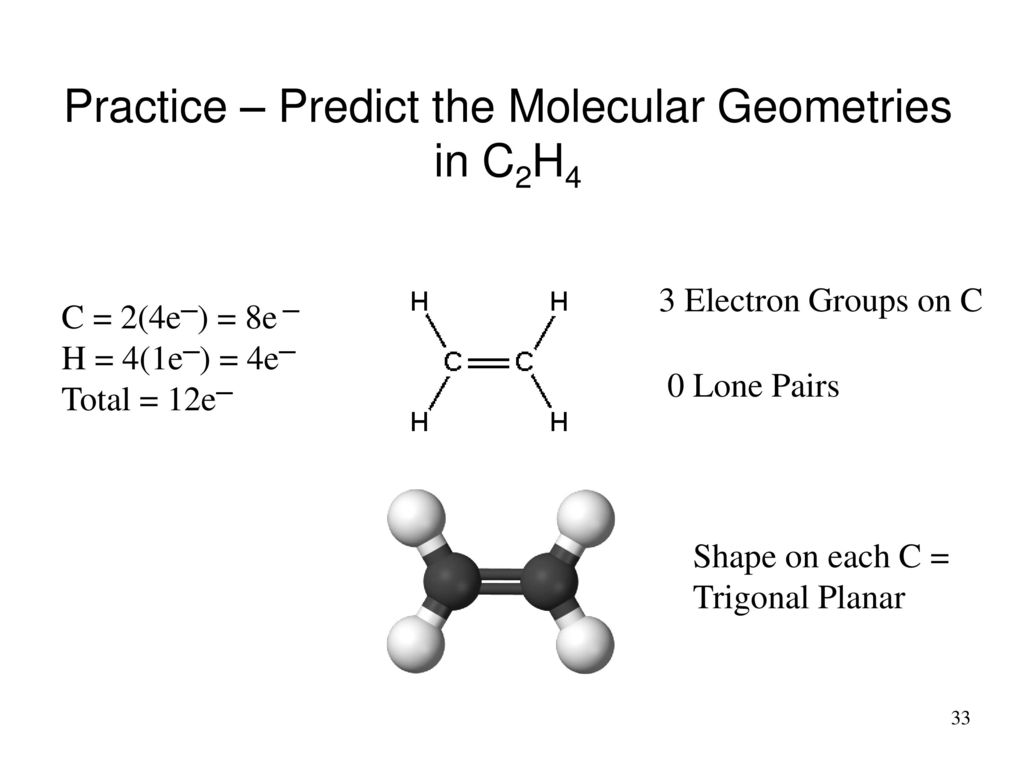
Molecular Geometry Predicted By Vsepr Ppt Download

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
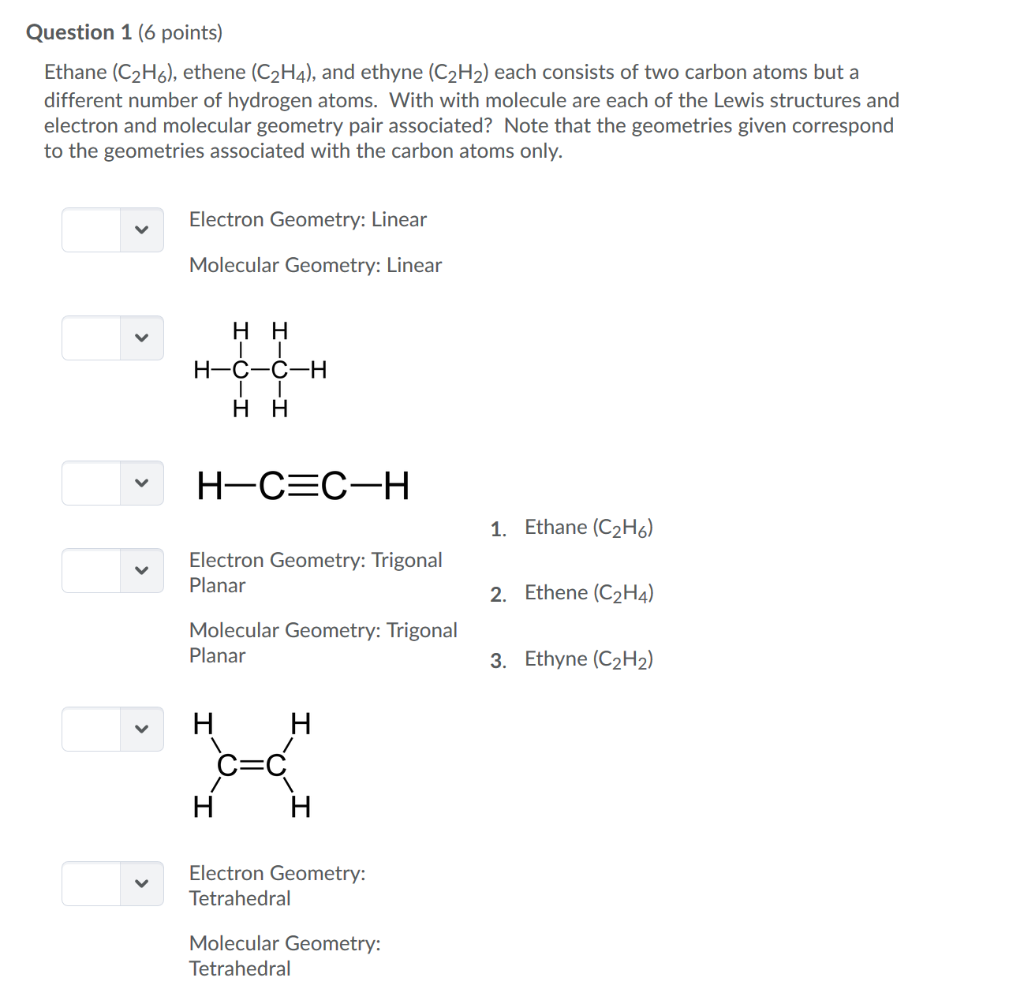
Question 1 6 Points Ethane C2h6 Ethene C2h4 Chegg Com
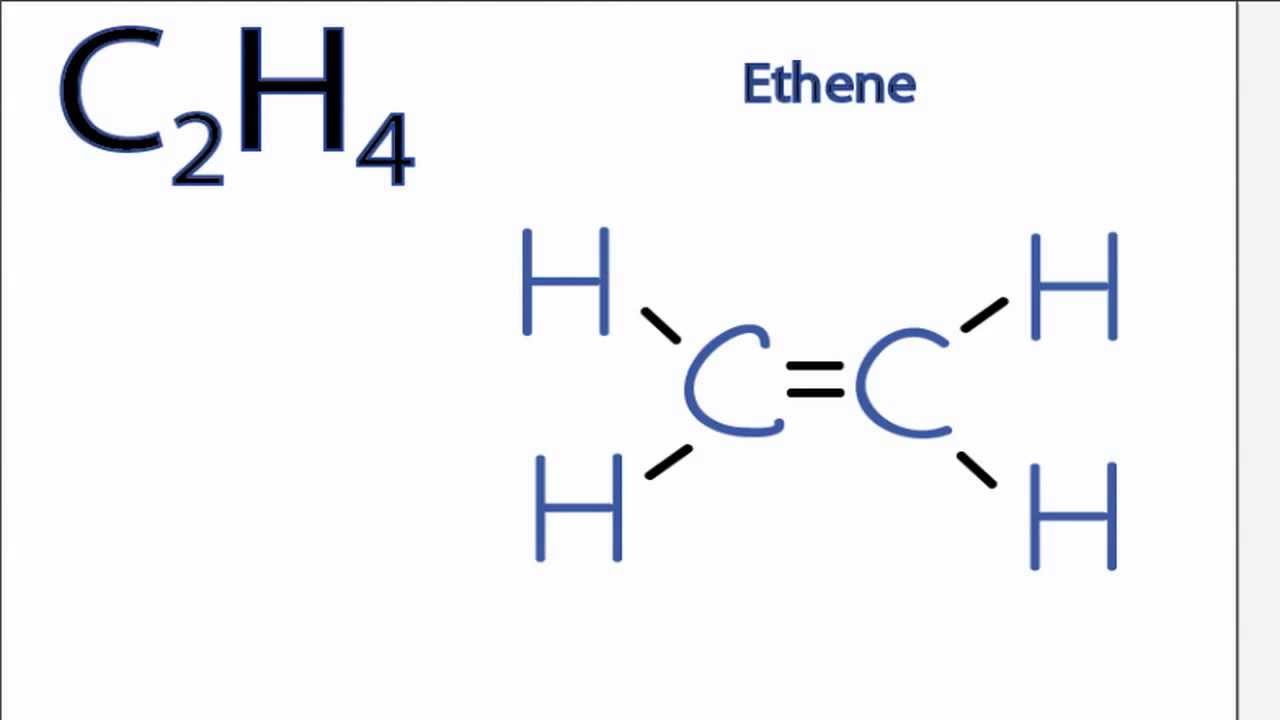
Is C2h4 Polar Or Nonpolar Youtube

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
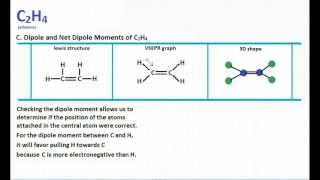
How To Draw Lewis Structure For C2h4 Drawing Easy

C2h6 Molecular Geometry Shape And Bond Angles Youtube
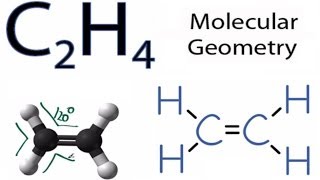
C2h4 Molecular Geometry Shape And Bond Angles Youtube

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity