Draw The Lewis Structure For Brf5
To draw the Lewis structure for a compound we must first calculate the number of valence electrons available to us. Draw The Lewis Structure For BrF5 In The Window Below And Then Answer The Questions That Follow.
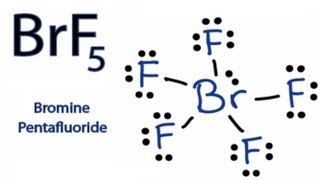
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
Well draw single bonds between the atoms for a total of 5 single bonds so 10 valence.
Draw the lewis structure for brf5. There are five Fluorine atoms and one Bromine. The hybridization on the Br is sp sp2 sp3 sp3d sp3d2. Draw the Lewis structure for BrF5 in the window below.
A step-by-step explanation of how to draw the BrF5 Lewis Dot Structure Bromine pentafluorideFor the BrF5 structure use the periodic table to find the tota. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons. Drawing the Lewis Structure for BrF5.
For BrF5 we have a total of 42 valence electrons. Many people forget to draw the lone pair of electrons so make sure to draw the lone pair of electrons. These electrons are called valence electrons.
Each element in the compound contributes a certain number of valence electrons from its outermost shell. Use information from step 4 and 5 to draw the lewis structure. Bromine is the least electronegative well put that in the center and then well put 5 Fluorines around the outside.
8 rows Follow some steps for drawing the lewis dot structure of BrF5. What is the hybridization on the Br atom. Is BrF5 Polar Or Nonpolar.
BrF 5 or Bromine Pentafluoride is a chemical compound that students might not come across frequently. Hybridization of BrF5 Bromine Pentafluoride Students before they learn about the hybridization of BrF5 should understand a few things about the chemical compound. BrF 5 is an interhalogen compound that comprises Bromine and Fluorine.
AsF3 CH3 BrF3 ClO3. When you begin drawing the structure 5 fluorine atoms will be drawn around the central atom bromine. Draw a valid Lewis structure showing all nonbonding electrons for dichloromethane CH2Cl2.
What is the anomaly in the Lewis dot structure of bromine pentafluoride BrF5. This problem has been solved. The molecule is choose one.
Draw the Lewis structure for BrF5. Count total valence. Lewis dot structure of BrF 5 Alternatively a dot method can be used to draw the lewis structure of BF 3.
After that draw the 3D structure for BrF5 using VSEPR rules. Drawing CH3I Lewis Structure is very easy to by using the following method. Bromine is the least electronegative well put that in the center and then well put 5 Fluorines around the outside.
C1 sp2 C2 sp3. Draw the Lewis structure for BrCl3. The electron geometry is octahedral and the hybridization is sp3d2.
A single bond will be drawn between each Br-F interaction which means already 10 valence electrons out of 42 available are exhausted. Draw lewis structure of BrF5 and XeF2 using VSPER theory. The polarity is best found by first drawing the Lewis dot structure for BrF5.
This is the BrF5 Lewis structure. What is the Lewis structure of BrF5. This is the BrF5 Lewis structure.
Determine the hybridization at each of the 2 labeled carbons. Next draw the 3-dimensional structure for BrF5. What is the hybridization on the Br atom.
For the BrF5 Lewis structure the total number. Draw the Lewis structure of acetic acid CH3CO2H clearly. The polarity is best determined by first of all drawing the lewis dot structure for BrF5.
Here in this post we described step by step method to construct CH3I Lewis Structure. Consider the molecule below. Provide a valid Lewis structure for a molecule with the molecular formula CH2O2.
Calculate the total valence electrons in BF 3 molecule. The carbon iodine and hydrogen elements come as the member of the carbon halogen and hydrogen family groups from the periodic table respectively. For BrF5 we have a total of 42 valence electrons.
Draw the Lewis structure for BrF5. Well draw single bonds between the atoms for a total of 5 single bonds so 10 valence electrons. A step-by-step explanation of how to draw the BrF Lewis Dot Structure Bromine FluorideFor the BrF Lewis structure calculate the total number of valence el.
By signing up youll get thousands of step-by-step solutions to your. Draw the Lewis structure of BrF5 and include the lone pairs.

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle
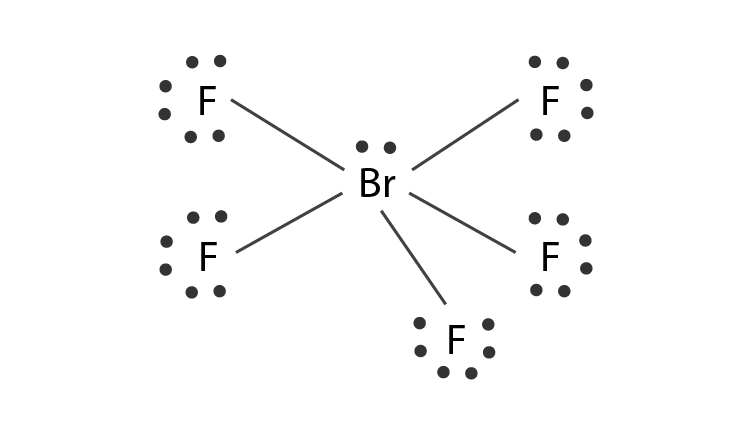
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Is Brf5 Polar Or Nonpolar Molecular Geometry Of Brf5

Bromine Pentafluoride Brf5 Lewis Dot Structure Youtube
Lewis Structure Of If5 Or Brf5 Ibr5 Icl5 Brcl5 Youtube

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville

Make A Sketch Of Brf5 Clutch Prep

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

The Central Atom In Brf5 Has How Many Bonding Pairs Of Electrons And How Many Non Bonding Pairs Of Electrons Study Com

Brf5 Lewis And 3 D Structure Dr Sundin Uw Platteville

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Bromine Pentafluoride Brf5 Is Sometimes Used As A Rocket P Clutch Prep

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube
Lewis Structure Of Brf5 Biochemhelp
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
How To Determine The Lewis Dot Structure For Bf4 Quora