H2so4 Lewis Dot Structure
Wallpapers h2so4 lewis dot structure. Merlic rules for drawing lewis structures 1.

Draw The Electron Dot Structure Of H2so4 Brainly In
I quickly take you through how to draw the Lewis Structure of H2SO4 Sulfuric Acid.
H2so4 lewis dot structure. Lewis Structure For S2Cl2. This means that the hydrogen atoms will be attached to the outside of the oxygen molecules. When we have an H or H2 in front of a polyatomic molecule like CO3 SO4 NO2 etc we know that its an acid.
Hence 322 given the result 16. A Lewis dot structure of an element is surrounded by the valence electrons of its atoms represented in the form of dots. Each dot represents a valence electron each line represents a bonded pair of electrons and each cl represents a.
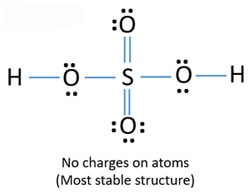
In HSO 4-Lewis structure Sulfur is least electron electronegative atom and goes in the center of the Lewis structure. Sulphur atom 2 8 6 is the central atom have six valence electrons as it has vacant d-orbital sulphur can show variable valencies. Let us consider the electron dot structure of sulphuric acid The chemical formula of Sulpuric acid is H 2 SO 4.
When we have an h or h2 in front of a polyatomic molecule like co 3 so 4 no 2 etc knowing this information makes it much easier to draw the lewis structure for h 2 so 4. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules. That means youre going to have an acid and that these hs are going to be attached to the outside of the oxygens.
Calcular el número de electrones de valencia. Draw The Lewis Structure For H2so4 lewis structure draw lewis structures acid sulfuric electrons valence structure resonance draw octet rule many bonded shapes each brainly lewis structure drawing begingroup stack lewis acid sulfuric draw structures bonded. For the H2SO4 molecule sulfur has the highest.
A step-by-step explanation of how to draw the H2SO4 Lewis Structure Sulfuric Acid. When we have an H or H2 in front of a polyatomic molecule like CO3. Lewis Structure of Sulfuric Acid H 2 SO 4 - Steps of Drawing.
This chemistry video tutorial explains how to draw the lewis structure of H2SO4 - Sulfuric AcidMy Website. Also note that you should put the HSO4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. H2so4 Lewis Dot Structure.
Sulfuric acid H 2 SO 4. To draw the Lewis Dot structure of the eqtextH_text2textStextO_text4 eq molecule let us go by step by step. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules.
I also go over hybridization shape and bond angles. This is the h2so4 lewis structure. A step -by step explanation of how to draw the h2so4 lewis structure sulfuric acidwhen we have an hor h2in front of a polyatomic molecule like co3so4no2etc we know that its an acid.
For the Lewis structure for HSO 4-you should take formal charges into account to find the best Lewis structure for the molecule. A step-by-step explanation of how to draw the K2SO4 Lewis Dot StructureFor K2SO4 we have an ionic compound and we need to take that into account when we dra. Oxygen and Sulfur atoms possess six electrons in their valence shells.
When we have an H or H2 in front of a polyatomic molecule like CO 3 SO 4 NO 2 etc we know that its an acid. In the H 2 SO 4 Lewis structure Sulfur is least electron electronegative atom and goes in the center of the Lewis structure. In the Lewis structure.
Lewis structure of sulfuric acid is drawn step by step in this tutorial. Sulfuric acid is a strong dibasic acid. Lewis structures commonly known as lewis dot diagrams or electron dot diagrams are representations of valence shell.
How to Draw the Lewis Structure for H2SO4. A dash or line is sometimes used to indicate a shared pair of electrons Both structures conform to the rules for lewis electron structures. Presenting molecular geometry on paper.
Note lone pairs can be converted into bonds until we get a stable structure. Lewis Dot Structure of H2SO4 Step 1 H2SO4 Valence Electrons. First we need to calculate the total number.
Total valence electrons concept is used to draw the lewis structure of H 2 SO 4. The key to understanding this lewis structure is that you have these hs in front and then you have this polyatomic ion. A step-by-step explanation of how to draw the H2SO4 Lewis Structure Sulfuric Acid.
It means the total pairs of electrons are 16.

Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

H2so4 Lewis Structure Sulfuric Acid Youtube
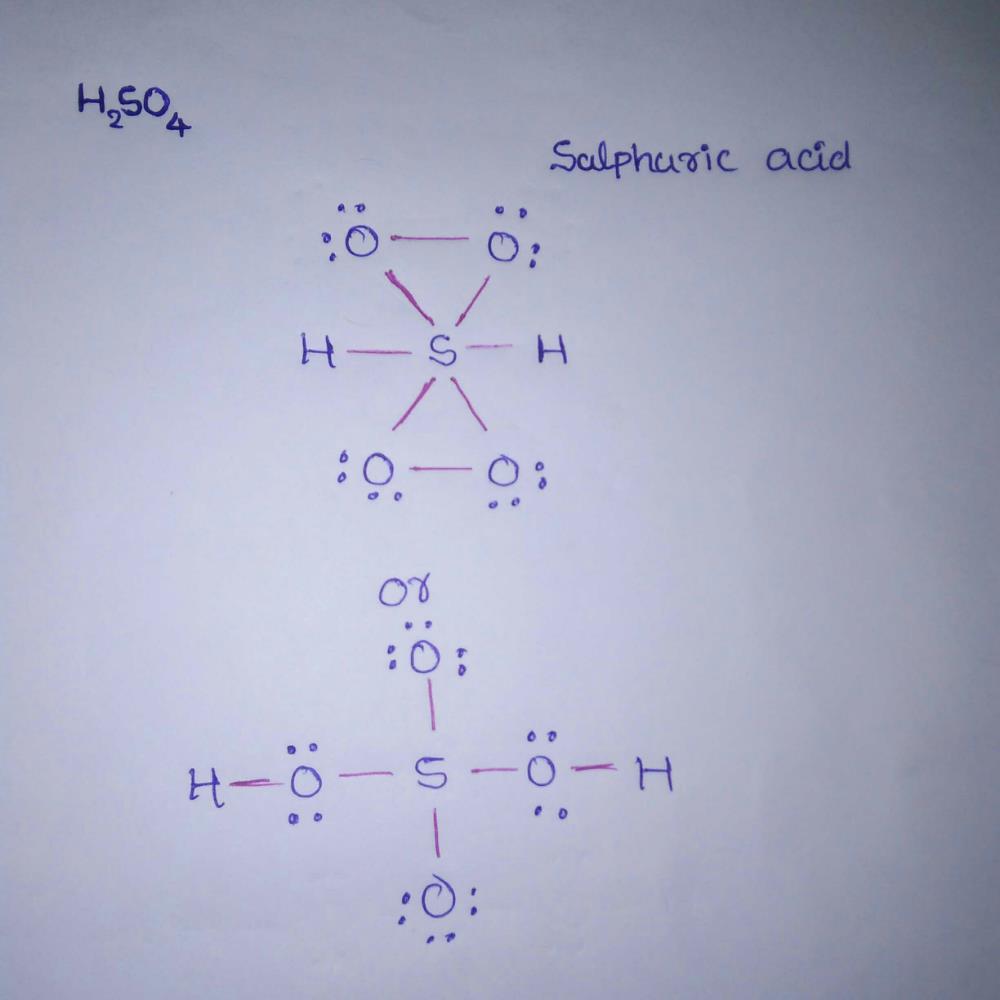
Electron Dot Structure Of Sulphuric Acid Pls Koi Bata Doo Edurev Class 11 Question
H2so4 Lewis Structure How To Draw The Lewis Structure For H2so4 دیدئو Dideo
I Draw Lewis Structure Of A Co3 2 B Nh4 Ions Ii Why H2so4 Has An Exception Lewis Structure Sarthaks Econnect Largest Online Education Community
What Is The Dot And Cross Structure Of Sulphuric Acid H2so4 Quora

Lewis Dot Structure Of H2so4 Brainly In
Lewis Dot Structure And Explanation Of H2so4 And Co Chemistry Topperlearning Com Dpr163cc

Draw The Lewis Dot Structures Of A H2so4 B Co3 2 C O3

H2so4 Lewis Structure Molecular Geometry And Hybridization Techiescientist

H2so4 Lewis Structure Molecular Geometry And Hybridization Techiescientist
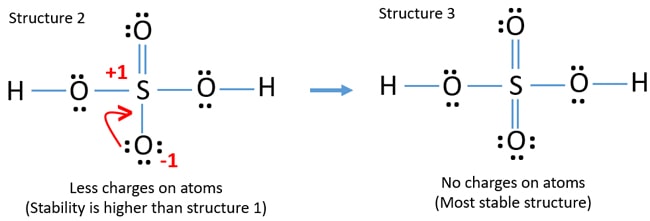
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
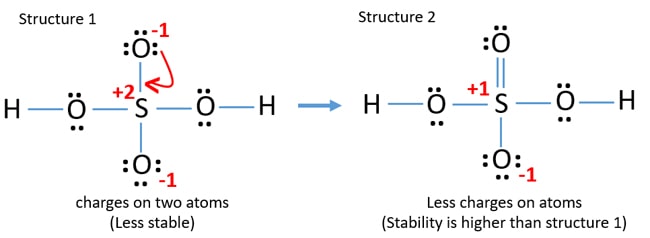
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
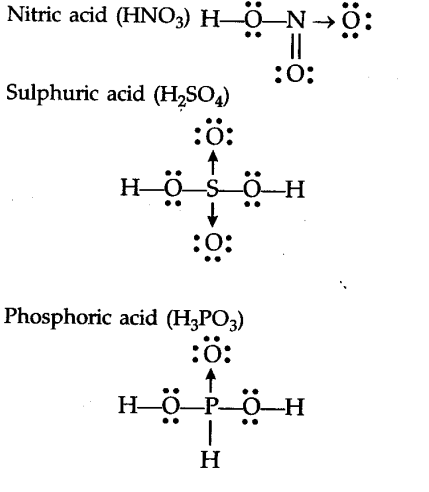
Draw The Lewis Dot Diagram Of Nitric Acid Sulphuric Acid And Phosphoric Acid Cbse Class 11 Chemistry Learn Cbse Forum
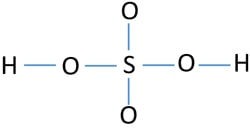
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

File Sulfuric Acid Lewis Png Wikimedia Commons

Sulfuric Acid Is The Industrial Chemical P Clutch Prep
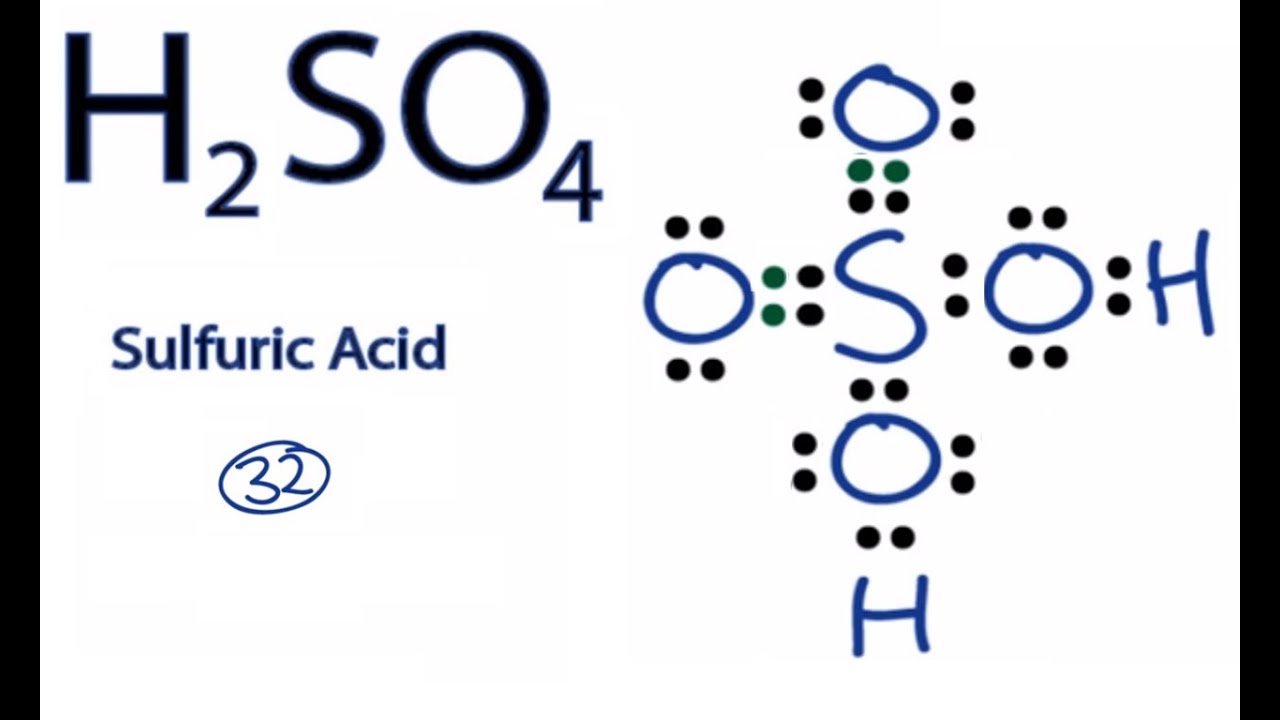
H2so4 Lewis Structure Molecular Geometry And Hybridization Techiescientist

