Hno3 Lewis Structure Resonance
The HNO3 Lewis structure is best thought of as the NO3 with an H attache. The Lewis structure is made by placing number of non-bonding electron around the symbol of element.

Consider The Lewis Structure For The Nitri Clutch Prep
Draw Lewis structure for one important resonance form of HNO3 HONO2.

Hno3 lewis structure resonance. However the answer then assumes that H N O X 2 has no resonance. Postby 705170809 Tue Nov 20 2018 1107 pm. This indicates that the ozone molecule is described by an average of the two Lewis.
In the water molecule AX2E2 the central atom is O and the Lewis electron dot formula predicts that there will be two pairs of nonbonding electrons. A molecule has resonance if more than one lewis structure can be drawn for that molecule. CWhat is the bond order of the equivalent N-O bonds in nitric acid.
If we draw it like the one on the right for hno3 in order to satisfy the octet rule the nitrogen atom would form 1 double bond and 2 single bonds. Since NO3- has resonance there are three different most stable structures but all. Which one of your structures.
For molecules with resonance each lewis structure individually does not accurately depict the structure of the molecule. More free chemistry help videos. Draw Lewis structure for one important resonance form of HNO3 HONO2.
HNO 3 Nitric acid Lewis Structure. A step-by-step explanation of how to draw the HNO3 Lewis Structure Nitric Acid. For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule.
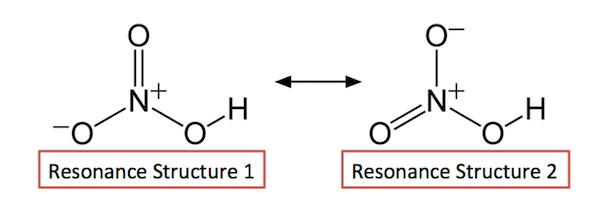
Lewis structure of nitric acid There is a NO bond in nitric acid lewis. This is because the real structure is known as a resonance hybrid. One of them however contributes much less to the resonance hybrid than the other two.
This is to say a molecule that is an intermediate of the two forms shown above. This is called resonance or mesomerism. 2nd structure shows 1 resonance structure of the nitrate ion of which there are 3.
As such is an important raw material for the chemical and pharmaceutical industry. Since the nitrogen dioxide ion has resonance the N O bonds are equal as resonance is in reality a hybrid of all of the possible structures for a certain molecule. 28 Nov 2020.
The Lewis dot structure of HNO3 H N O 3 including all three resonance structures are shown below. Thats with the electrons. HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element.
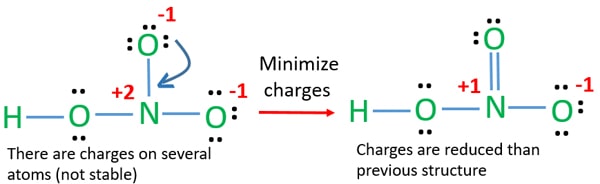
This is a pattern seen with many acids. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom.
Sketch the three resonance structures and assign a formal charge to each atom. For HNO3 in order to satisfy the octet rule the nitrogen atom would form 1 double bond and 2 single bonds. Let us consider the case of the Lewis electron dot structure of nitric acid HNO3 HNO 3 Lewis structure.
A question on the 1996 AP Chemistry Free Response asks. Drawing the Lewis Structure for HNO 3. Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written.
After determining how many valence electrons there are in HNO3 place them around the central atom to. DIn which species HNO3 or NO3- are the N-O bond s longer. Lewis structure of NO 3- ion is important because it is required to draw resonance structures of nitrate.
The N O bonds in the N O X 2 X ion are equal in length whereas they are unequal in H N O X 2. A Draw Lewis Structures for Nitric Acid HNO3 NO2OH. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms.
Nitric acid HNO 3 has three resonance structures. Lewis Structure for NO3-. Nitric acid is a strong oxidizing agent and it dissolves practically all metals except gold and platinum and some other precious metals.
Unlike O3 though the actual structure of CO32 is an average of three resonance structures. Include all lone pair electrons. Equivalent Lewis structures are called resonance structures or resonance forms The correct way to describe ozone as a Lewis structure would be.
This problem has been solved. Include all lone pair electrons. Is co3 2 a resonance structure.
And can you explain why that depiction is the only correct one. Resonance structures of NO 3- ion Lets draw the three resonance structures for the nitrate anion NO 3- Lone pairs charges and bonds of NO 3- ion. However the first two resonance structures are significantly more favorable than the third because they have smaller amount of formal charges.
The actual distribution of electrons the resonance hybrid is an average of the distribution indicated by the individual Lewis structures the resonance. We cannot describe the structure of HNO3 with our system of drawing molecules Lewis system as shown above. NO3- has resonance which allows all the bond angles to be the same between the different resonance structures since resonance doesnt move the actual formation of the atoms but only rearranges the double bonds.
If there are equivalent resonance structures draw all of them. Based on octet rule alone there are 3 possible resonance structures that are favorable.

Consider The Lewis Structure For The Nitri Clutch Prep

Simple Procedure For Writing Lewis Structures Lewis Structures For Iodate Ion Io3 Chemistry Net Chemistry Writing Lewis
Hno3 Nitric Acid Lewis Structure

Hno3 Nitric Acid Lewis Structure
In Lewis Structure Of Hno3 Does Nitrogen Share Two Electrons With Two Of The Oxygens Do Those Oxygens Share Or Not Share Electrons With The Nitrogen Quora

The Lewis Structure Of Hno3 Chemistry Stack Exchange

Draw A Lewis Structure For Nitric Acid The Hydrogen Atom Is Attached To One Of The Oxygen Atoms Brainly Com

Chemistry Net Carbocation Rearrangements And Change In Ring Size Chemistry Organic Chemistry Math Equations

Chemistry Net Carbocation Rearrangements Chemistry Advanced Organic Chemistry Organic Chemistry Reactions

Nitric Acid Hno3 Lewis Structure Properties Uses Rankred

The Lewis Structure Of Hno3 Chemistry Stack Exchange

The Lewis Structure Of Hno3 Chemistry Stack Exchange

Consider The Lewis Structure For The Nitri Clutch Prep

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom