In The Lewis Dot Structure For Nh3 How Many Dots Are On The Nitrogen
Log in sign up. 27 Lewis Dot Diagram For N.

How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube
Ditulis oleh Anonim Selasa 25 Mei 2021 Tambah Komentar.
In the lewis dot structure for nh3 how many dots are on the nitrogen. Calculate the total valence electrons in the molecule. The Lewis structure of NH3 is made in such a manner that the scarcity of one valence electron in each hydrogen atom total three hydrogen atoms as well as three valence electrons in the nitrogen atom is fulfilled and balanced. To draw the NH3 Lewis structure we have to find out the NH3 total valence electrons firstWe express valence electrons as dots in lewis dot structure.
Alternatively a dot method can be used to draw the NH 3 Lewis structure. Ammonia or Nitrogen trihydride. This means that there are three bonded atoms and one lone pairfor a coordination number of four around the nitrogen the same as occurs in H2O.
Remember that uncharged nitrogen has 3 bonds and one lone pair and hydrogen has one bond and no lone pairs. On the periodic table nitrogen is in group 5 or 15 so it has 5 valence electrons and then hydrogen is in group 1. The Lewis dot structurefor ammonia NH3.
Lewis structure of NH 3. Step-by-step tutorial for drawing the Lewis Structure for Ammonia. Each hydrogen atom is covalently bonded to the nitrogen via an electron pair and another pair of electrons is attached to the nitrogen.
What is the lewis dot. So far weve used six of the NH3 Lewis structures total 8 outermost valence shell electrons. In the Lewis dot structure for HCN how many bonds are between carbon and nitrogen.
Electron dot diagram for nh3. N 71s²2s²2p³ The highest value of principal quantum number here is n2. A step by step explanation of how to draw the n3 lewis dot structure.
Ammonia or nitrogen trihydride. A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas - Diatomic NitrogenFor the N2 structure use the periodic table to find t. The nitrogen looks like.
On the periodic table Nitrogen is in group 5 or 15 so it has 5 valence electrons and then Hydrogen is in group 1. I show you where Nitrogen is on the periodic table and how to determine. Put nitrogen in center and arrange hydrogen atoms on the sides.
We also have a handy video on the 5 things you need to know for general chemistry. The lewis dot structure for nh3 ammonia is shown above. You could also represent the bonds as dots between the two atoms but this may be confused with the lone pair electrons on the nitrogen.
One lone pair of electrons on the nitrogen atom in the tetrahedral geometry of the NH3 molecule. H n h h on top of the nitrogen where there isnt a hydrogen are the two other electrons. Complete the middle nitrogen atom stability and if necessary apply a covalent bond.
A step-by-step explanation of how to write the Lewis Dot Structure for NH3 Ammonia or Nitrogen Trihydride. Lewis dot structure for nh3. Calculate the total valence electrons in the molecule.
The electron-dot structureof NH3places one pairof nonbonding electronsin the valence shell of the nitrogen atom. Lewis Dot Structure of Molecules. A step-by-step explanation of how to draw the N2O2 Lewis Dot StructureFor the N2O2 structure use the periodic table to find the total number of valence elec.
NH3 commonly known as ammonia is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities. The Lewis structure for NH3 isThe Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash each to an H atom. Arrange electrons until nitrogen gets 8.
In the lewis dot structure for nh3 how many dots are on the nitrogen. 3 Calculate the percent ionic character of a chemical bond between calcium and oxygen. Were going to do the Lewis structure for NH3.
Ammonia or nitrogen trihydride. How Many Valence Electrons Does Nitrogen Have Socratic. To get the valence electrons of nitrogenwe need to look at the electronic configuration of nitrogen.
You could also represent the bonds as dots between the two atoms but this may be confused with the lone pair electrons on the nitrogen. A step-by-step explanation of how to draw the Lewis dot structure for N Nitrogen. The N atom then has two dots on the unconnected side.
It has one valence electron but we have 3 Hydrogens so lets mutiply that. A step-by-step explanation of how to draw the NH3 Lewis Dot Structure AmmoniaFor the NH3 structure use the periodic table to find the total number of vale. The number of dots equals the number of valence electrons in the atom.

Lewis Dot Structures And The Octet Rule
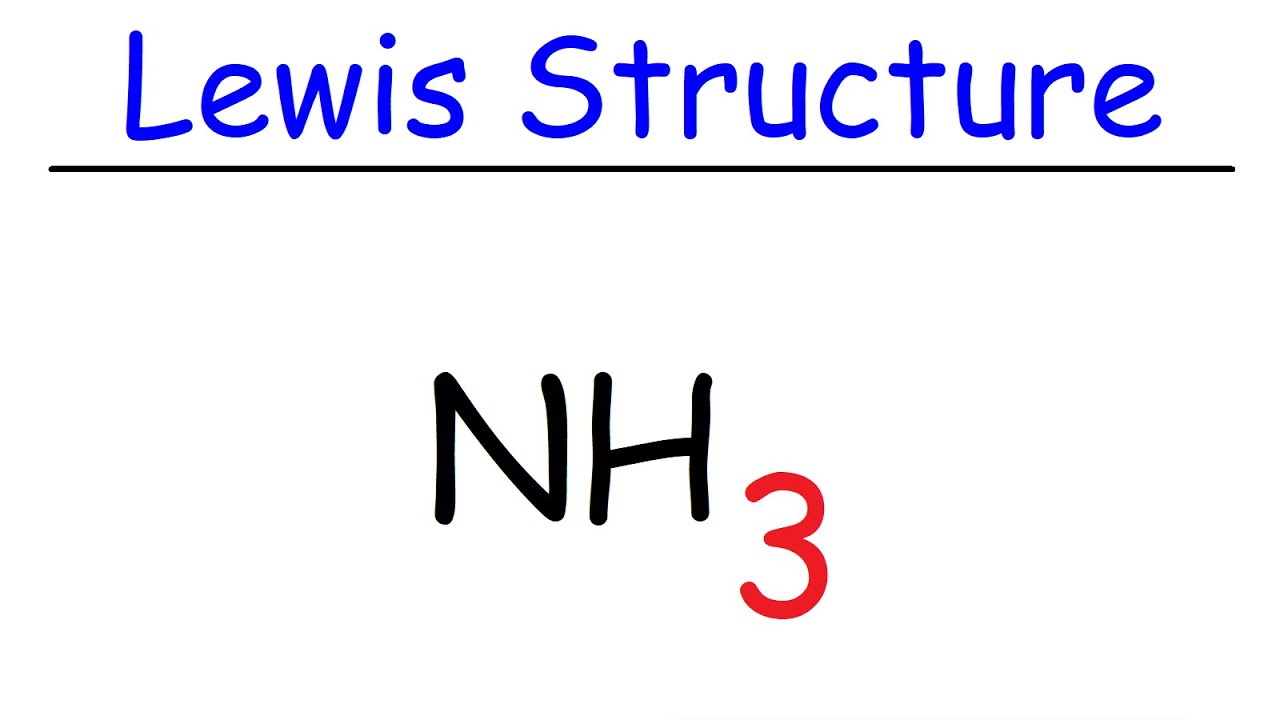
Nh3 Lewis Structure Ammonia Youtube

Lewis Dot Structure For Nitrogen Atom N Youtube
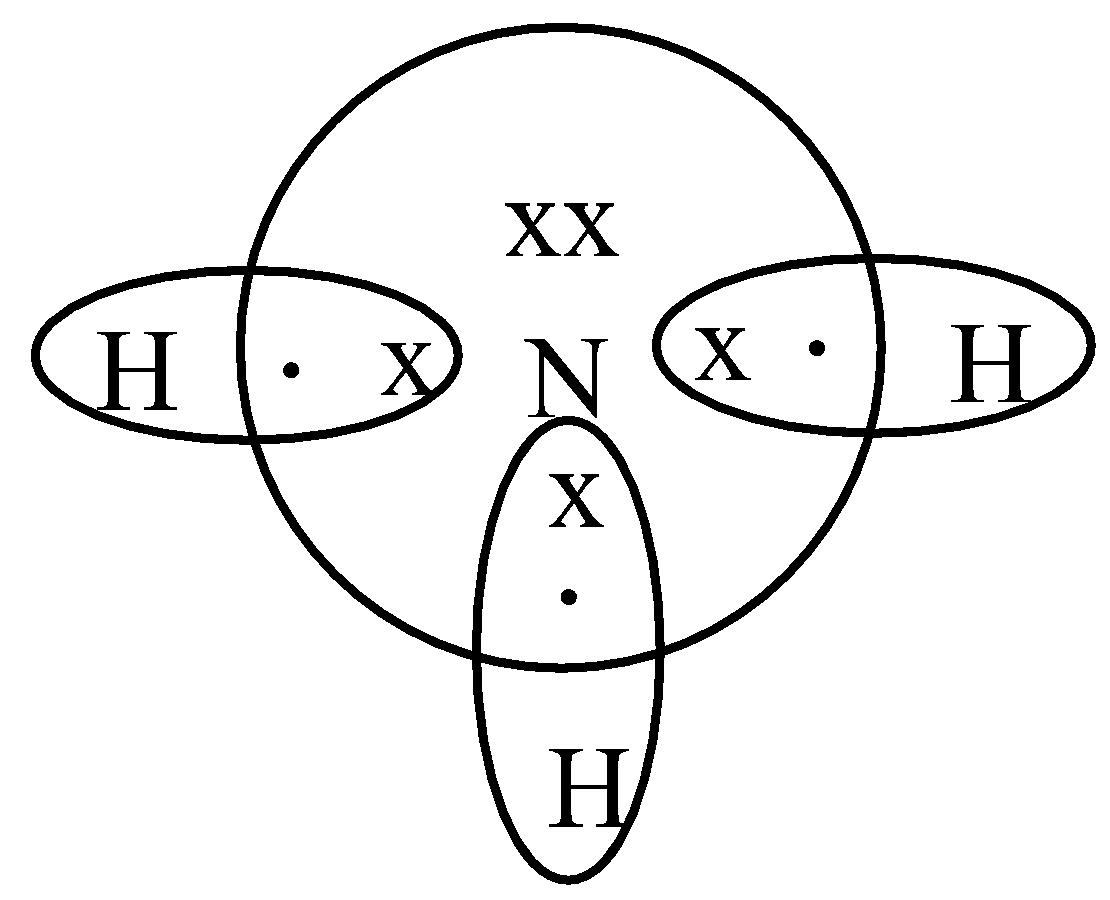
Draw An Electron Dot Diagram To Show The Formation Class 11 Chemistry Cbse

Lewis Dot Structure For Argon Atom Ar Youtube

How Is The Lewis Dot Diagram For Nh3 Determined Quora
How To Know Where To Put The Dots On A Lewis Structure Quora

Lewis Electron Dot Structures Detailed Explanation With Examples Videos
Does It Matter Where You Put The Dots On A Lewis Structure Quora

Lewis Dot Structure Easy Hard Science
How Is The Lewis Dot Diagram For Nh3 Determined Quora

Lewis Dot Structure Easy Hard Science
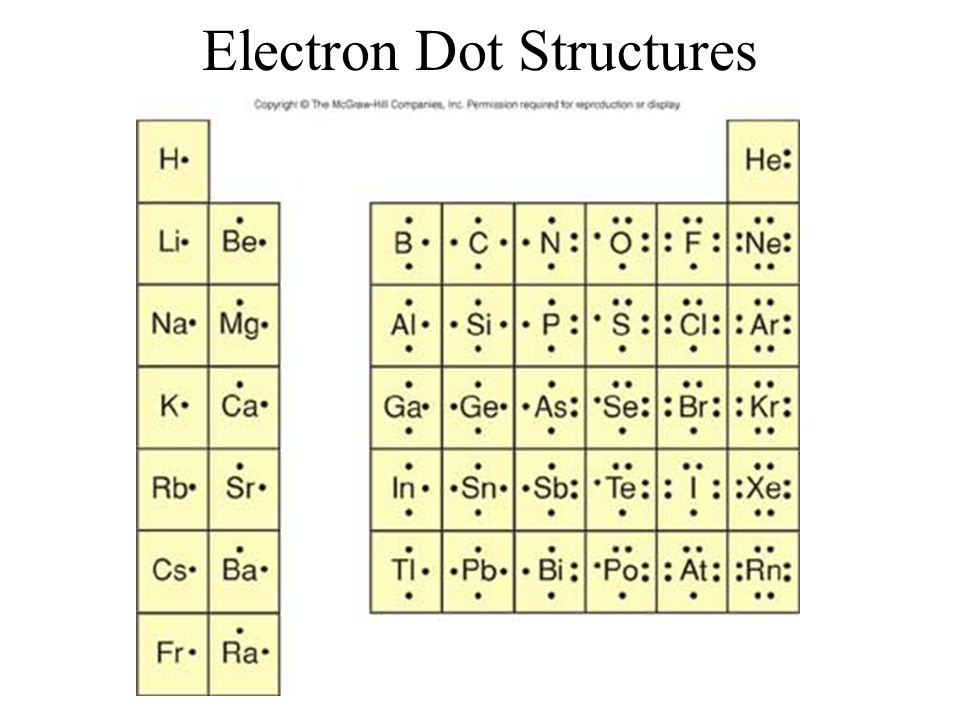
Electron Dot Structures Ppt Video Online Download

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science
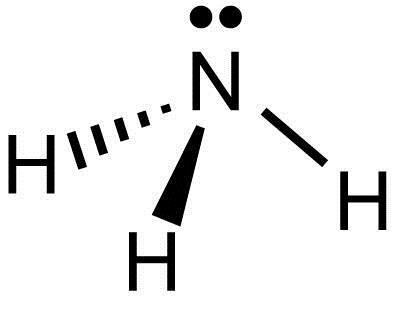
What Is The Lewis Structure Of Nh3 Socratic

Lecture 8 2 Lewis Dot Structures For Molecules
How To Know Where To Put The Dots On A Lewis Structure Quora
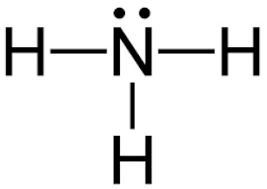
By Looking At The Lewis Dot Structure Of Ammonia Nh3 Class 11 Chemistry Cbse