Lewis Structure For C2h4 Polar Or Nonpolar
NOodd number of e Structural Isomers. The formal charge on carbon in CF4 is zero.

Is C2h4 Polar Or Nonpolar Techiescientist
Is c2h4 polar or nonpolar simple is that c2h4 is a nonpolar molecule.

Lewis structure for c2h4 polar or nonpolar. If you look at the Lewis structure for C2H4 it appears to be a symmetrical molecule. It has a colorless flammable gas with a musky and sweet odor. Draw the electron-dot structure for C2H4.
Another reason is that the hydrogen-carbon bonds are nonpolar because of nearly the same electronegativity. HCN C2H6 NH4 C2H4 NO3- СН. CaC2 2H2O Ca OH2 C2H2.
Is it polar or nonpolar. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. However to determine if CH4 is polar we consider the molecula.
As a result the dipole of the molecule of Ethylene turns out to be zero. CF4 is nonpolar in nature but the bond present in it is polar. It is a chemical formula for Ethylene or Ethene.
Polarity polar nonpolar polar polar polar. The molecular geometry of C2H4 is trigonal planar above are the explanation of its Lewis structure. The chemical equation for the same is as follows.
Is C2H4 polar or nonpolar. If you look at the lewis structure for ch4 it appears to be a symmetrical molecule. What Is The Lewis Dot Structure VSEPR Geometry Molecular Geometry And Are They Polar Or Nonpolar For The Following.
Is the molecule polar or nonpolar. What is the electronic geomet. When we say it has the most simple structure it is because only two types.
In the next part you will learn a little about structural isomers and how to draw them. Is c2h4 polar or nonpolar simple is that c2h4 is a nonpolar molecule. Another reason is that the hydrogen-carbon bonds are nonpolar because of nearly the same electronegativity.
What is its molecular shape. However to determine if C2H4 is polar we consider the molecular geomet. Nonoctet Molecule Name Lewis Dot Structure Polar or Shape Number of Valence Electrons Nonpolar.
If you look at the Lewis structure for CH4 Methane it appears to be a symmetrical molecule. To determine the polarity of Ethane we will first need to look at is Lewis Structure. Nonpolar covalent bonds with equal sharing of the bond electrons arise when the electronegativities.
Moreover as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals the hybridization of. Ethylene C2H4 has sp2 hybridization and its bond angle is between 1166 to 1217. The molecular geometry of CF4 is tetrahedral and electron geometry is also tetrahedral.
The difference in electrostatic potential is also minimal giving an overall nonpolar molecule. So Is C2H4 Polar or Nonpolar. Polar and nonpolar molecules are the two broad classes of molecules.
The polarity of ch4 is non polar. I hope its now clear to you that C2H4 is a nonpolar molecule because of the same electronegativity of both atoms. We have also shared a detailed blog on the Lewis Dot Structure of Ethane previously which you can check out if you want to find out the number of valence electrons and the process of making C2H6 Lewis Structure step-by-step.
CF4 is a nonpolar molecule. Draw the lewis structure for se h. Draw the Lewis structure for C2H6.
HO SO32- SiH SO42- NF. The resultant molecular structure for acetylene is linear with a triple bond between the two carbon atoms one sigma and two pi-bonds and a single sigma bond between the carbon and hydrogen atoms. The net dipole moment of carbon tetrafluoride is zero.
As a result the dipole of the molecule of Ethylene turns out to be zero. By signing up youll get thousands of. What is the name for ClO3.
If you look at the lewis structure for ch4 methane it appears. Fill in the table. Total 8 bonded electrons present in CF4 lewis dot structure.
CH4 CO2 CO unusual SO CHCI. Ethylene C2H4 is nonpolar in nature because of the symmetrical linear geometrical shape. The bond angle of CF4 is 1095º.
Molecular polarity polar or nonpolar. The Lewis structure of the methane CH4 molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. C2H6 is a saturated hydrocarbon named as ethane.
Ethylene C2H4 is nonpolar in nature because of the symmetrical linear geometrical shape. C2h4 has two ch and four h molecules.

Methane Molecule Showing Covalent Bonding Dot And Cross Covalent Bonding Structural Formula Molecules
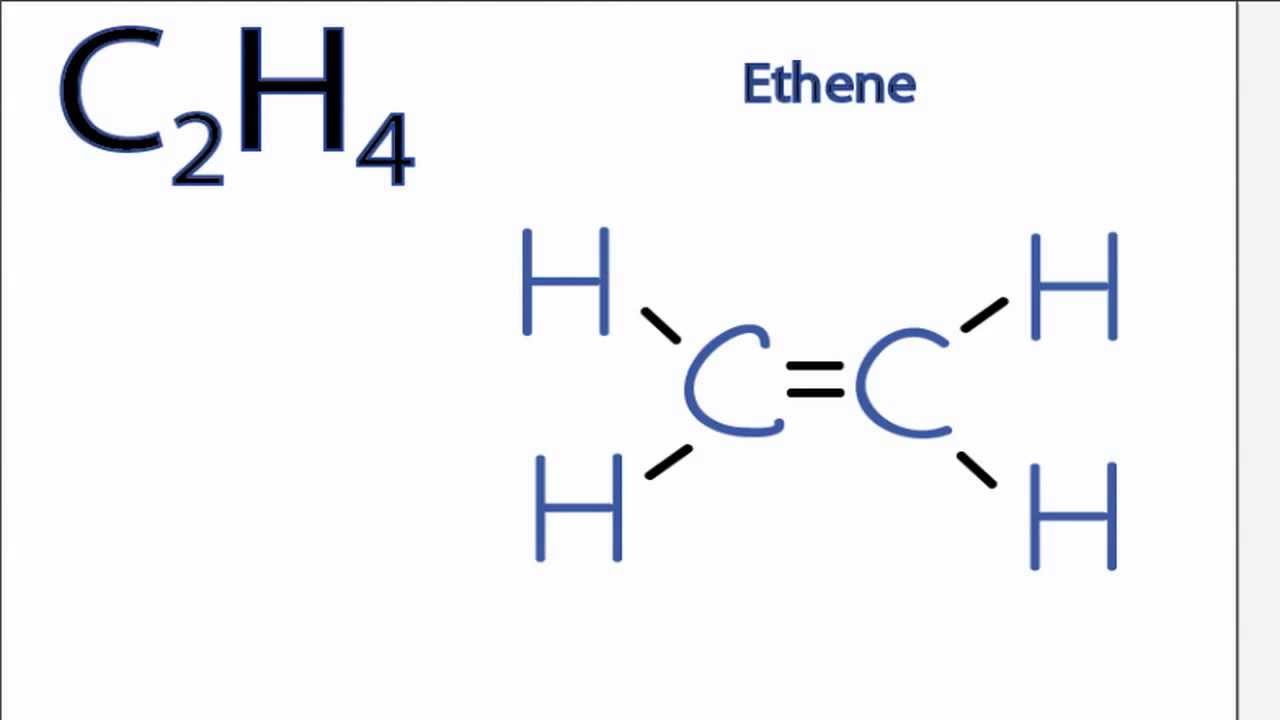
Is C2h4 Polar Or Nonpolar Youtube

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity

Dipole Moment And Symmetry Affect Boiling Point And Melting Point Organic Chemistry Chemistry Chemistry Notes

The Relative Strength Of Hydrogen Bonding Dipole Dipole Interactions And London Dispersion Forces Organic Chemistry Chemistry Intermolecular Force

Organic Chemistry Notes Chemistrynotes Com Chemistry Notes Organic Chemistry Notes Organic Chemistry

Bond Lengths And Bond Strengths For Sp3 Sp2 Sp C H Bonds Bond Length Bond Chemistry

Hybridization Ethylene Organic Chemistry Molecular Geometry Chemistry

Curved Arrow Cannot Start From A Positive Sign Chemistry Curved Arrow Covalent Bonding

Is C2h4 Polar Or Non Polar Ethylene Youtube

Sp3 Sp2 And Sp Hybridization In Organic Chemistry With Practice Problems Chemistry Steps Organic Chemistry Chemistry Molecular Geometry

Curved Arrows And Rules For Drawing Resonance Structures Chemistry Curved Arrow Organic Chemistry

Melting Point Of Butyul Alcohol Isomers Organic Chemistry Chemistry Chemical Bond

Dipole Moment Bond Moment Group Moment And Influence Of Dipole Moment Chemsolve Net Organic Chemistry Books Covalent Bonding Physical And Chemical Properties

Dipole Moment Bond Moment Group Moment And Influence Of Dipole Moment Chemsolve Net Organic Chemistry Books Covalent Bonding Physical And Chemical Properties

Is C2h4 Polar Or Non Polar Ethylene Youtube

