Lewis Structure Of Ethylene C2h4
8C 4H 12 Valence Electrons. 1 Show answers Another question on Chemistry.

Hybridization Structure Of Ethylene Mcc Organic Chemistry
Matches the chemical name of each oxide of phosphorus to its chemical formula.

Lewis structure of ethylene c2h4. Electron Dot Structure for ethane C2H4. Ethylene CH2CH2 or C2H4 CID 6325 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Draw the Lewis structure for the ethylene C2H4 molecule.
It consists of two carbon molecules and 4 hydrogen molecules. Lets take a look. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
The clouds are grey and ground is wet. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. This means that the carbon atoms share 4 electrons.
Lewis dot structure of C 2 H 4. To get the valence electrons of carbonwe need to look at the electronic configuration of carbon. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule.
Therefore the total number of valence electrons in Ethylene C 2 H 4. It is a chemical formula for Ethylene or Ethene. Count total valence electron in C2H4.
It has 1 valence electron. C 61s²2s²2p² The highest value of principal quantum number here is n2. Be sure to include all resonance structures that satisfy the octet rule.
Ethene is an unsaturated hydrocarbon. If we come way over here to Hydrogen its in group 1. To do that we always count our valence electrons up first.
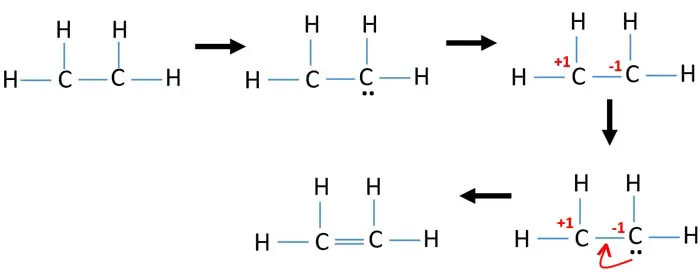
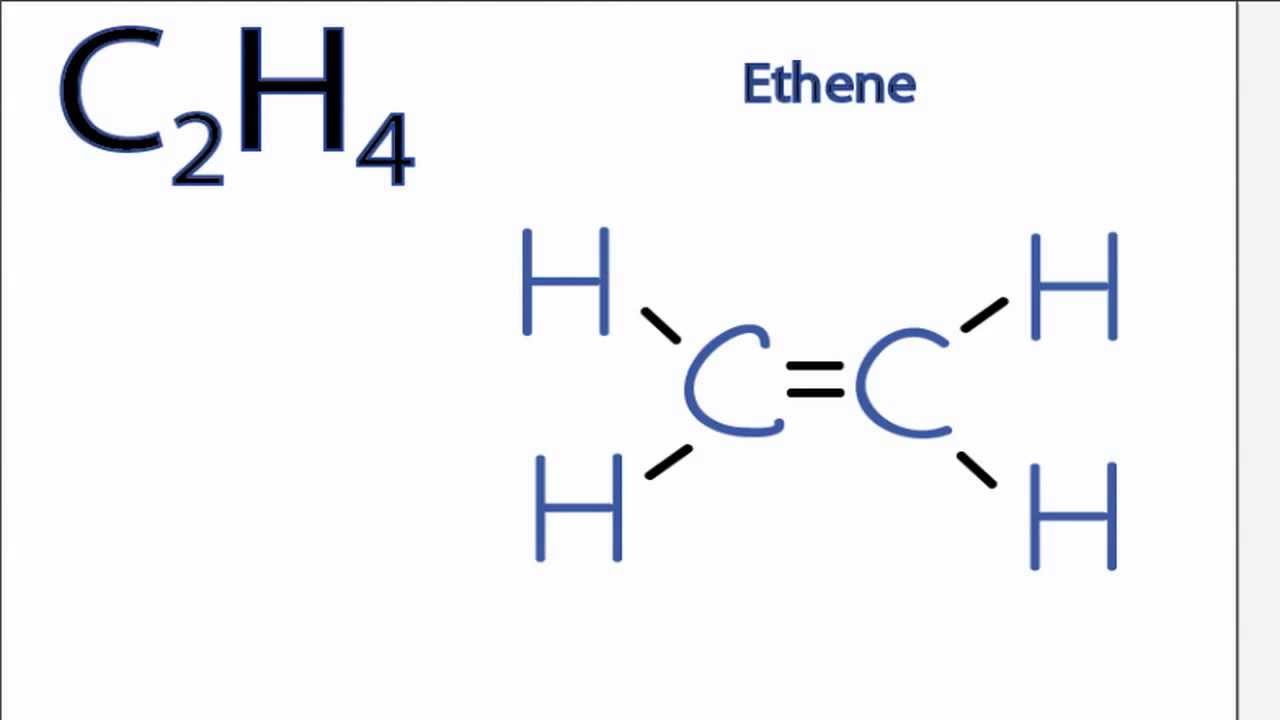
We have 12 available valence electrons. No lone pair is present on the central or outer atom in the lewis structure of ethene. Ethene C 2 H 4 Lewis Structure Hybridization.
To draw the C2H4 lewis structure we have to find out the C2H4 valence electrons firstWe express valence electrons as dots in CH2CH2 lewis dot structure. Use information from step 4 and 5 to draw the lewis structure. What Is The Hybridization Of C In Ethylene.
Ethylene-13C2 C2H4 CID 12242921 - structure chemical names physical and chemical properties classification patents literature biological activities safety. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Which is the correct Lewis structure for ethylene C2H4. We review their content and use your feedback to keep the quality high. A Draw The Lewis Structure For Ethylene C2H4 B List The Electronic And Molecular Geometries For C2H4 Around The Central C Atom the C Atoms Are Equivalent.
The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. Carbon is in group 4 sometimes written 14 so it has 4 valence electrons. Most stable structure is taken as the lewis structure of ethene.
Experts are tested by Chegg as specialists in their subject area. For C 2 H 4 you have a total of 12 total valence electrons. Fill In The Orbital Diagram Of Unhybridized Valence.
Ethenes lewis structure can be built by VSEPR rule. Hydrogen is the least electronegative element here. However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure.
There are two triangles overlapping each other as we can see in the diagram. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. Calculate the total valence electrons in the molecule.
The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1. Alternatively a dot method can be used to draw the lewis structure. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure. The electron dot structure is drawn using Lewis-dot structure. The key to understanding how to distribute the valence electrons is to.
It has double bond present between two carbon atoms. Hybridization of atoms in ethene molecue can be found from lewis structure. Its C2H4 and we want to write the dot structures for ethene.

Structure And Bonding In Ethene The Pi Bond Chemistry Libretexts

Draw The Lewis Structure For Ethylene C2h Clutch Prep
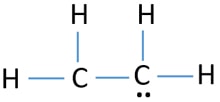
Ethene C2h4 Lewis Structure Hybridization

Ethene C2h4 Lewis Structure Hybridization

C2h4 Molecular Geometry Shape And Bond Angles Youtube
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Write The Electron Dot Structure Of Ethene Molecule C2h4
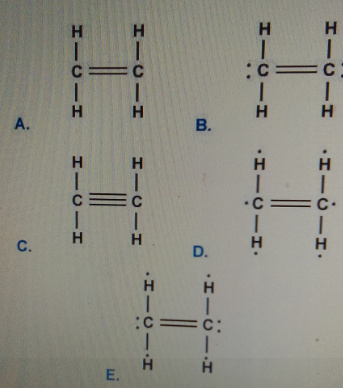
Which Is The Correct Lewis Structure For Ethylene C2h4 Home Work Help Learn Cbse Forum

Valence Bond Theory Boundless Chemistry
Is C2h4 Polar Or Nonpolar Youtube

How Many Sigma And Pi Bonds Are Present In Ethene Quora
Lewis Structure Of C2h4 Biochemhelp

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In
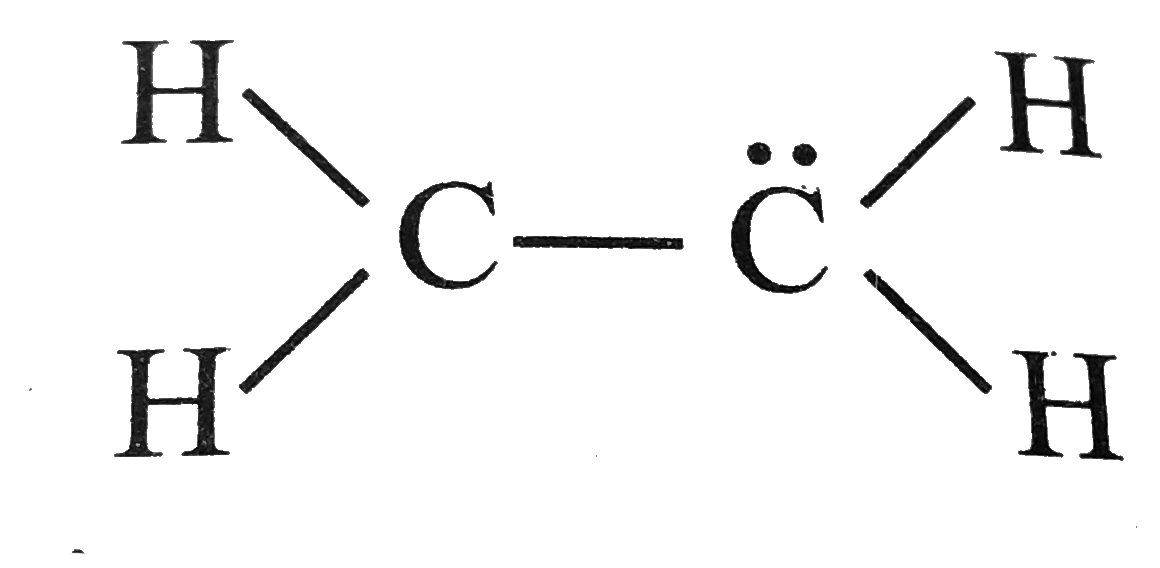
Write Lewis Structure For The Following A Ethene C 2 H 4 The Most Important Reactant In Polymer Manufacture B Nitrogen N 2 The Most Abundant Atmosheric Gas C Methanol Ch 4 O An Important Industrial Alcohol

6 2 Lewis Structures Introductory Chemistry

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
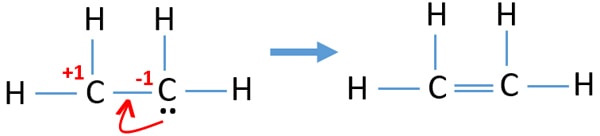
Ethene C2h4 Lewis Structure Hybridization