(nh4)2so4 Dissociation In Water Equation
Writing Dissociation Equations for Strong Electrolytes Soluble. Calculate the pH of an aqueous solution that is 085 M in ammonia.

Solution Chemistry Chp 7 8 Ppt Download
When NH4Cl dissolves in water it dissociates into its cation NH4 and anion Cl-.

(nh4)2so4 dissociation in water equation. Include the ratio of moles underneath each formula. The molar mass of ammonia is 1703 gmol. It can therefore be used to.
The dissociation of ammonium ion in water Dissociation Reaction Examples. Write an equation to represent the dissociation of propionic acid CH3CH2COOH in water. The ionic strength of the water is 96B1 2 NH 3 NH 4 NH 2.
The dissociation of NH42SO4 forms 2 moles of ammonium ions NH4 and 1 mole of sulfate ion SO42-. The compound ammonium sulfate NH42SO4 is soluble in water. The PBE for the system of HCl in water is Although H3O is formed from two reactions it is included only once in the PBE.
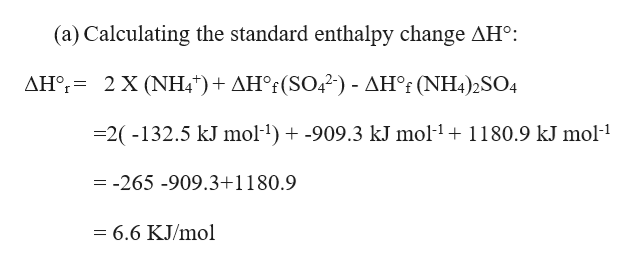
NH4Cl H2O - H2O NH4 Cl - 156K views. ΔHf NH42SO4s -11809 kJ mol-1. Mole NH4 2 x 05 1and 05 mole SO4-2.
Type your answer using the format H2O for H2O and NH4 for NH4. Write the dissociation equation for MgOH2 Magnesium hydroxide MgSO4 Magnesium sulfate Please show how you came to the answer so that I can better understand how to do it. The balanced dissociation for for NH42SO4 is.
Write equations for the dissociation. 2 NH42SO4 2NH4 SO4 2- The valency of sullphate SO4 2- anion is 2 and not 1. Use uppercase for the first character in the element and.
When it is in the solution it forms ions. I get so lost on conversions and mols and all of that Ive asked for help 2 NH42SO4 2NH4 SO4 2- The valency of sullphate SO4 2. ΔHf NH4aq -1325 kJ mol-1.
1HClaq HaqClaq After successful dissociation of HClin water. NH_4OHaq - NH_4aq OH-aq When ammonium hydroxide is dissolved in water the ion-water attraction overcomes the attraction between ions so it dissociates into the ammonium cation and hydroxide anion. NH42SO4s ------2NH4aq SO42-aq a Calculate the standard enthalpy change ΔH for this reaction using the following data.
Some of the dissolved ammonia gas NH3 reacted with the water to form the aqueous ammonia ions NH4. Do not include states in your answer. Write The K Expression For A Generalized Reaction.
The pKa term is the negative log of the acid dissociation constant. Solved Expert Answer to a Write the dissociation equation for solid ammonium sulfate NH42SO4s in water. So when it is dissolved we get this equation.
Use the pull-down boxes to specify states such as aq or 8 но Submit Answer Retry. When you write a dissociation reaction you separate the two ions place their charges above their symbols and then balance the entire equation. Mass balance equation Charge balance equation.
In an experiment ammonia gas NH3g was bubbled through distilled water. The dissociation of NH42SO4 when it is dissolved in water is given as. Use the lowest possible coefficients Chemistry.
NH42SO4 2NH4 SO4-2 05 mole NH42SO4 must produce 1. 1 Write the equation for the dissolution of each of the following in water and then determine the number of moles of each ion produced as well as the total number of moles of ions produced. Suggest specific experiments to specify whether it is a complex or double salt.
NH42SO4s 2 NH4 aq SO42- aq Comments 3 where do get the 2 from tho. Base dissociation of ammonia is as follows- NH3 H. The salt ammonium sulfate dissolves in water according to the reaction.
ΔHf SO42-aq -9093 kJ mol-1. Write equations for the dissociation of the following in water. I get so lost on conversions and mols and all of that Ive asked for help 2 NH42SO4 2NH4 SO4 2- The valency of sullphate SO4 2- anion is 2 and not 1.
Potassium alum KAlSO4 2 12H2 Ois obtained in the form of octahedral crystals when a solution of K2 SO4 and Al2 SO4 3 is concentrated by evaporation. In this video we will describe the equation NH42SO4 H2O and write what happens when NH42SO4 is dissolved in waterWhen NH42SO4 is dissolved in H2O w. In the presence of water an ionic moiety dissociates into cations and anions as follows.
NH42SO4 is an ionic compound that dissociates itself upon its addition to the solvent to form a solution. Write the net ionic equation for the dissociation reaction that occurs when solid ammonium sulfate dissolves in water. Write the dissociation equation for each ionic compound in solution.
Writing dissociation equations when substance is dissolved in water littlelacy Tue 09252007 - 1811 So I have to write a bunch of dissociation equations when certain substances are dissolved in water. Eqrm ABs rightarrow Aaq B-aq eq. Write a chemical equation for the dissociation of the hypochlorite ion ClO-1 in water to make a basic solution.
1 The Ionic Solid Nh4 2so4 Is A Strong Electrolyte Chegg Com

Ppt Types Of Aqueous Reactions Powerpoint Presentation Free Download Id 3850851

Is Nh4 2so4 Soluble Or Insoluble In Water Youtube

Is Nh4 2so4 Acidic Basic Or Neutral Dissolved In Water Youtube

Chapter 9 Chemical Change Ppt Download
Answered The Salt Ammonium Sulfate Dissolves In Bartleby

2 Ammonium Sulfate Dissociate According To The Chegg Com
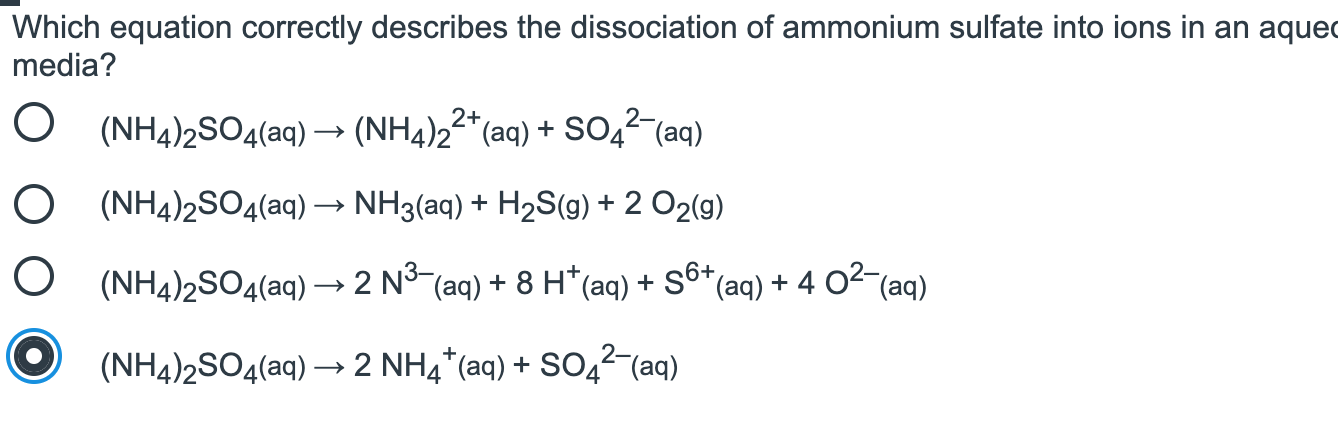
Which Equation Correctly Describes The Dissociation Chegg Com

Ppt Chapter 4 Powerpoint Presentation Free Download Id 2948816
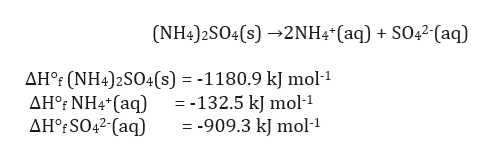
Answered The Salt Ammonium Sulfate Dissolves In Bartleby

Equation For Nh4 2so4 H2o Ammonium Sulfate Water Youtube
Equation For Nh4 2so4 H2o Ammonium Sulfate Water Youtube
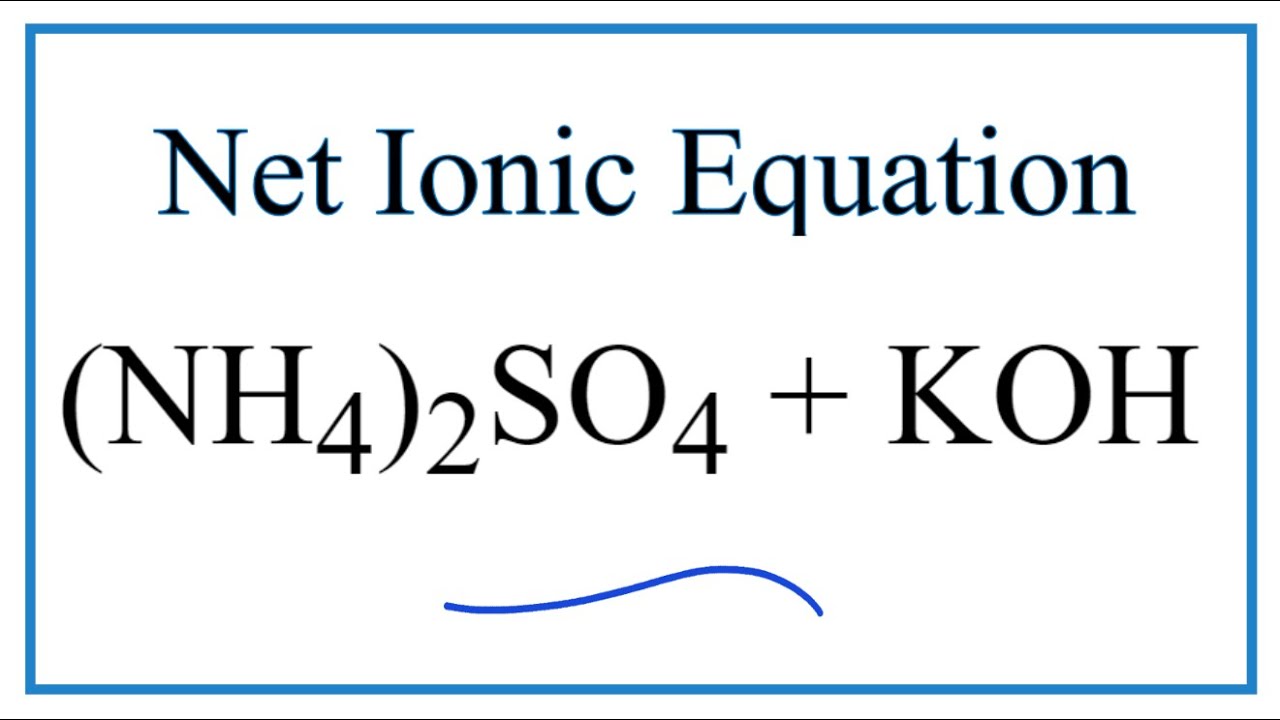
How To Write The Net Ionic Equation For Nh4 2so4 Koh K2so4 Nh3 H2o Youtube

Answered The Salt Ammonium Sulfate Dissolves In Bartleby

How To Write The Net Ionic Equation For Nh4 2so4 Naoh Na2so4 Nh3 H2o Youtube

The Compous Ammonium Sulfate Nh4 2so4 Is Soluble In Chegg Com

How To Balance H2so4 Nh4oh Nh4 2so4 H2o Sulfuric Acid Ammonium Hydroxide Youtube

The Molecular Nature Of Matter And Change Ppt Download

How To Write The Net Ionic Equation For Nh4oh H2so4 Nh4 2so4 H2o Youtube