So3 2- Lewis Structure Resonance
The more resonance structures the less reactivity. Polysulfur is an elemental sulfur.
.jpg)
Lewis Structure For So32 Sulfite Ion Resonance Structures
There are seven resonance structures for SO_3.
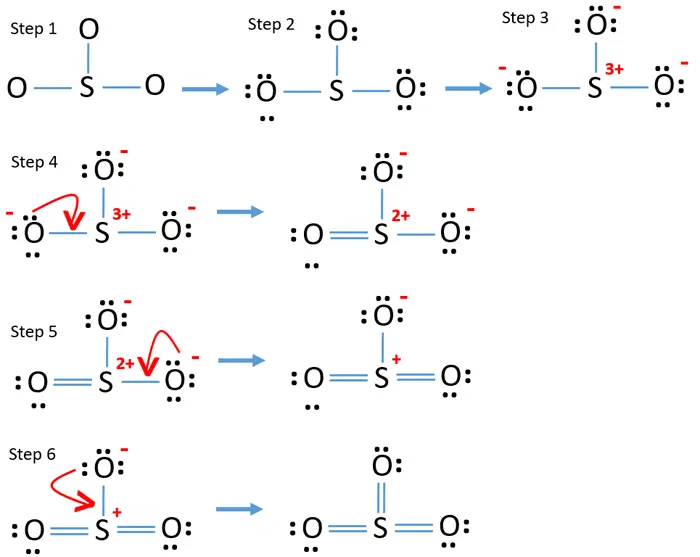
So3 2- lewis structure resonance. In the picture you can see the Lewis representation of sulfate ion and their different resonance structures SO₃². Sulfur does not have an expanded octet in SO3 you dont need to know why. Lets do the SO3 2- Lewis structure.
- lewis structure drawing - bonding electrons - nonbonding electrons - hybridization - AXE notation - molecular geometry - polar or nonpolar - resonance - isomers - wedge and dash drawing. By signing up youll get thousands of step-by-step solutions to your. There are three resonance structures SO3 Sulfur trioxide.
But there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2. To develop the resonance structures it is necessary to practice and practice. E SO3 2 I dont understand what this question is really talking about.
The global idea is to move electrons from more negative atoms to the less ones but there are no specific rules. So we have 26. Show all equivalent resonance structures and on any one of the resonance structures show the formal charge on each atom.
For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up here--there are two extra valence electrons. It has a role as an agrochemical an antifungal agent and a Saccharomyces cerevisiae metabolite. There are three major resonance structures for SO3.
Each oxygen atom has two lone pairs in SO 3 lewis structure. The Lewis Dot Structure for SO 3 2-. Therefore the lewis dot structures will actually be very different.
Combining resonance structures 2 and 3 results in the best overall description of the bonding for the molecule. SO3 does not have the extra 2 electrons SO3 2- has. Complete the following for BrF3 SF4 IF4 SO3-2 XeF2 and SF2.
Lewis Dot of the Sulfite Ion. What is the Lewis dot structure of SO3. C I3 D SO3.
Charges of S make structures 2 and 3 more stable or more important contributors. We start with a valid Lewis structure and then follow these general rules. Therefore five electron groups are around the central atom of SO32- ion.
These are the equivalent structures where there exist one SO bond and two SO bonds. I also go over hybridization shape and. SO 4 2-Lewis Structure Sulfate ion.
Lewis structure of sulfate ion is drawn in this tutorial step by step. A SCO C central atom B BF3. For the SO3 2- Lewis structure the total number of valence electrons.
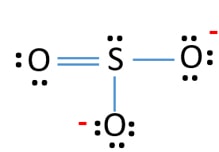
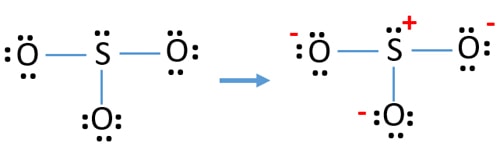
When you draw the ion with all single bonds and 3 lone pairs on each oxygen there is a formal charge of 1 on S and -1 on each O as shown. I received a question in a plf regarding the lewis structure of the sulfite ion SO32- so to clarify. It is a conjugate base of a trisulfanide.
Note that SO3 is a bi. The fourth resonance structure with three SO bonds is noncontributory due to reasons beyond the scope of MCAT chemistry. There are three double bonds around sulfur atom with oxygen atoms in SO molecule.
In which one of the following is the best Lewis structure a resonance structure. The sulfite anion SO3 2- is present in wines and is used as preservative in certain foods. Lewis structure provides the bonding present between atoms of a molecule and lone pair of electrons involved in a molecule.
Explain the resonance structures for the sulfite ion SO32-. Sulfur brings 6 and oxygen brings 3 each. We can find out the structure of molecule with the help of this structure.
In each of them S has a formal charge of 2 and two of the O atoms have formal charges of -1. When you draw the Lewis structure you first get the three structures at the top. Central atom of SO32- ion is sulfur.
Lets put the Sulfur at the center and the Oxygens around the outside. In each of the three structures in the middle S has a formal charge of 1 and one of the O atoms has a formal charge of -1. Total valence electrons concept is used to draw the lewis structure of SO 4 2-In lewis structure of sulfate ion there should be charges on several atoms due to -2 charge.
This is also a possible resonance structure although the octet rule is not satisfied. What is the Lewis dot structure of SO3 2. Hybridization of SO 3 molecule.
The formal charges for all atoms are zero for structure 4 given earlier. As shown below The lewis structure of SO3 is being considered when no electrons. Trisulfide 2- is a triatomic sulfur.
Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO32- ion.
How Is The Hybridization Of So3 2 Determined Quora
Lewis Structure For So32 Sulfite Ion Resonance Structures
So3 Lewis Structure Sulfur Trioxide Youtube
Lewis Structure For So32 Sulfite Ion Resonance Structures
What Are All Resonance Structures For So3 Socratic

What Are The Resonance Structures For So3 Quora

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora

How Many Electron Dots Are In The Lewis Structure Of So 3 2 Study Com

Lewis Structure For So32 Sulfite Ion Resonance Structures

Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps

View 23 Resonance Structures For So3 2

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

So3 2 Lewis Structure And Molecular Geometry Youtube
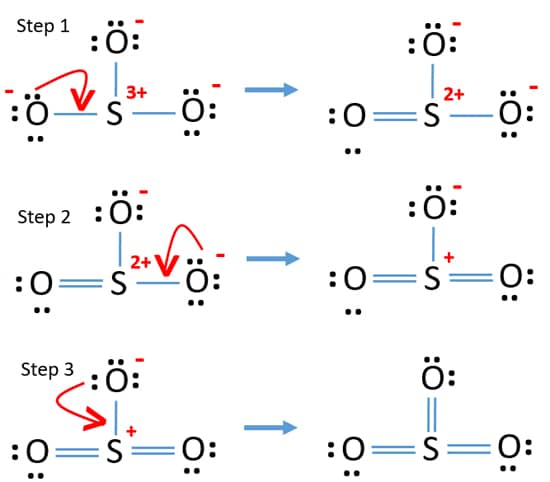
Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

Explain The Resonance Structures For The Sulfite Ion So32 Brainly Com

Lewis Structure For So32 Sulfite Ion Resonance Structures