The Lewis Structure Of Pf5 Is Given Below. What Is The Hybridization Of The Phosphorus Atom
Thus the Lewis structure of any compound can be formed using these simple steps. The central phosphorus atom has eqsp3d eq atomic orbital hybridization which accounts for its ability to form five single bonds to the fluorines.

Pf5 Molecular Geometry Shape And Bond Angles Youtube
The Hybridization of IF5 is Sp3d2.
The lewis structure of pf5 is given below. what is the hybridization of the phosphorus atom. In PF_5 the valence electrons of phosphorus are 5. H 6 Sp3d2 hybridization. It will form single bonds with the five fluorine atoms.
Calculate the total number of valence electrons present. P less electronegative atom than S central atom. These will account for 10 of the 40 the molecule has.
The hybridization of a molecule can be understood in two ways-The Theoretical part The central atom Iodine atom has 7 valence electrons. The molecules Lewis structure will look like this. They are inclined at an angle of 90 degrees to one another.
In PF5 phosphorus has 5 bond pair and 0 lone pair so steric no is 5. What is the hybridization on the fluorine atom. The phosphorus atom will be the molecules central atom.
Determine the central atom in this molecule. PF 5 Hybridization The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3 but when it is in an excited state the electrons from 3s orbital get unpaired. The Lewis structure of PF 5 is The electronic configuration of P 15 is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3.
The first valence electron of the halogen. Añd shape of PF5 is Trigonal bi pyramydal TBP. H always goes outside.
The Lewis structure of PF5 is given below. Our videos prepare you to succeed in your college classes. What is the hybridization on the fluorine atom.
Each fluorine atom will have three lone pairs of electrons which will account for the rest of the. H 5 Sp3d hybridization. And we take the oxidation state of halogens as 1.
Of electrons is 5. Its electronic configuration in the ground state is I 5s2 5p5. Find the total valence electrons for the molecule.
The Lewis structure of PF5 is given below. XeO 2 F 2. Let us help you simplify your studying.
H 2 S NCl 3 OH -. Therefore from phosphorus and fluorine 5-50 electrons 0 lone pairs. Sp3d2 hybridization has 1s 3p and 2d orbitals that undergo intermixing to form 6 identical sp3d2 hybrid orbitals.
We are being asked to identify the hybrid orbitals used by phosphorus P or the hybridization of P in PS 2-. Based on the Lewis structure In PF 5 there five electron domains around the P atom. Steps for Writing Lewis Structures.
Lewis structure of PO 4 3-ion. 3 hydrogen atoms are bonded to oxygen so the number of the monovalent atoms M 3. The hybridization of PF_5 is sp3d.
The Phosphorus Pentafluoride PF5 has sp3 hybridization because of its trigonal bipyramidal shape. So for one F electron is 1 and for F_5 the no. PBr5 has Phosphorous as the central atom has eight electrons in its outer shell after forming the bond with neighbouring halogen atoms.
In the lewis structure of PO 4 3- three is a double bond between phosphorous atom and one oxygen atomBetween other oxygen atoms there are only single bonds with phosphorous atom. Thus by the formula V 6. Pe sp2 hybrid spºd hybrid sp3 hybrid sp hybrid spd.
In PF5 the phosphorus has 0 lone pair and 5. Key Features of. If steric no is 5 hybridization is sp3d.
There are 5 sigma bonds in this compound. Hybridization in Phosphorus pentachloride PCl 5 sp 3 d 2 Hybridization. These 6 orbitals are directed towards the corners of an octahedron.
You can also calculate hybridization by counting the bond and lone pair that give you the steric number. Put the least electronegative atom in the center. NOCl CF 2 Cl 2 HCN.
There are no lone pairs in the Lewis Structure of PF 5 and there are five single bonds between Phosphorus and Fluorine atoms. As the Phosphorus forms bonds with five Bromine molecules all the electrons enter in different orbitals of the shell. If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back.
It is easy to understand the hybridization of the molecule after knowing its Lewis structure. First we will have to draw the Lewis Structure of PS 2-. Now lets find the hybridization of H3O using this formula In hydronium ion the central atom is oxygen and it has 6 valence electrons.
Our videos will help you understand concepts solve your homework and do great on your exams. Pe sp2 hybrid spºd hybrid sp3 hybrid sp hybrid spd. Also each oxygen atom has a -1 charge.
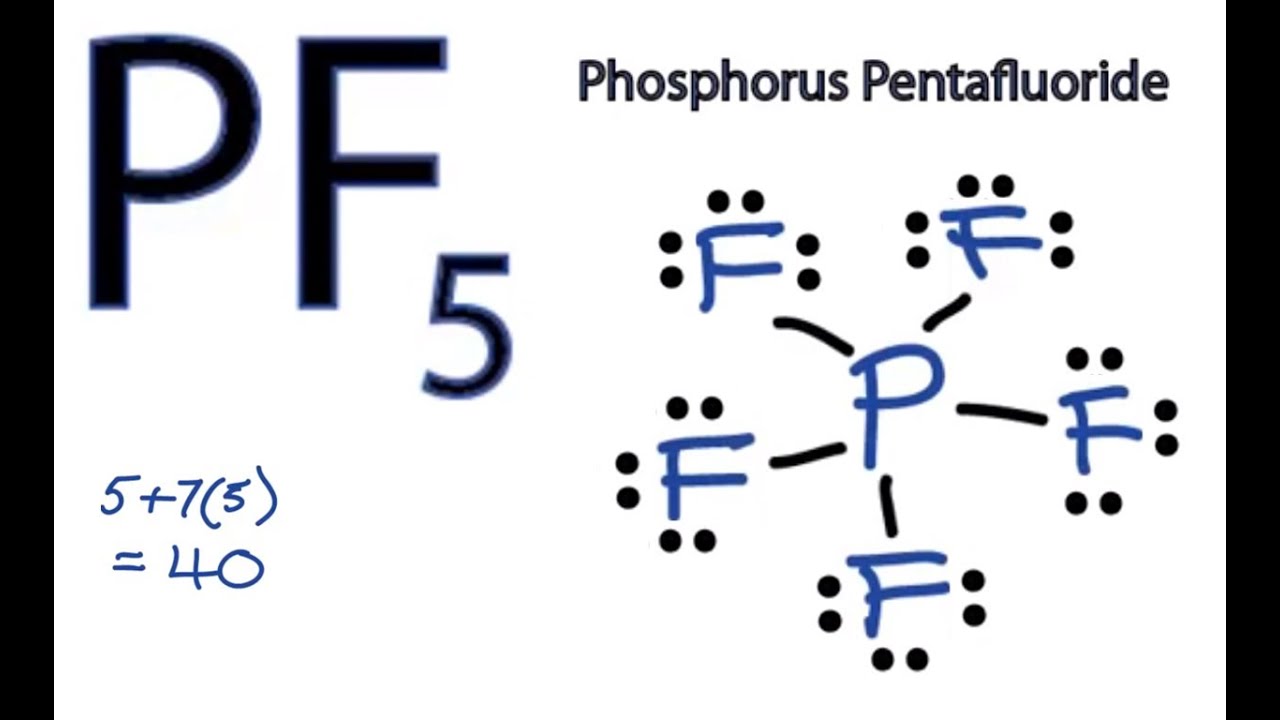
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube

Chapter 2 Molecular Structure And Bonding

Is Pf5 Polar Or Nonpolar Techiescientist
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
What Is The Lewis Structure Of Pcl5 Quora

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora

What Is The Hybridization Of Mathjax Fullwidth False Pf 6 Explain Study Com

How Is The Phosphorus Atom Hybridized In The Phosphoric Acid Molecule A Sp 3d 2 B Sp 2 C Sp 3d D Sp 3 Study Com
What Is The Structure Of Pf5 And How Can We Explain Its Geometry Quora

Is Pf5 Polar Or Nonpolar All About Pf5 Polarity Shape

Is Pf5 Polar Or Nonpolar Techiescientist
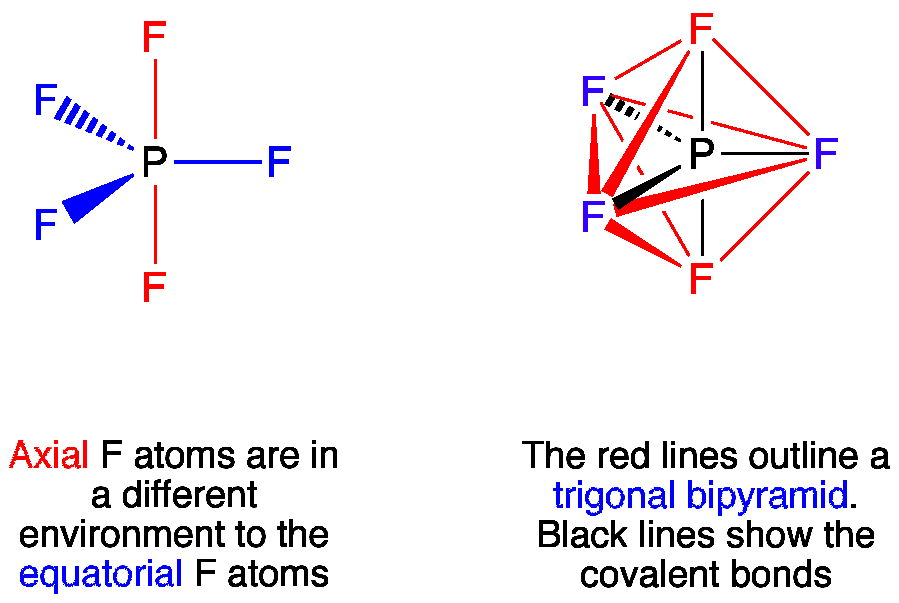
Vsepr Pf5 Phosphorus Pentafluoride

Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube

Pf6 Lewis Structure How To Draw The Lewis Structure For Hexafluorophosphate Youtube

Pf5 Lewis Structure And Molecular Geometry Made Easy Youtube
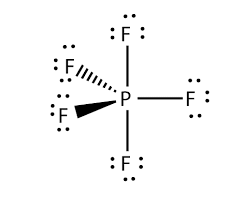
Pf5 Lewis Structure Molecular Geometry Bond Angle And Shape Geometry Of Molecules
