What Is The Molecular Geometry Of Sef4
What shape is sef4. It forms a see-saw shape and has a trigonal bipyramidal molecular geometry.

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube
It has a molecular geometry of the formula AX4E.
What is the molecular geometry of sef4. Equatorial-equatorial 120. You can watch a video on trigonal bipyramidal geometry here. Here the quotient is 4 and remainder is 2.
XeF4 Molecular Geometry It is easier to understand the molecular geometry of a given molecule once we know its Lewis structure. Regarding this what is the molecular geometry of SeF4. A CIF5 b CLO2 c TeCL 2 d PCI3 e SeF4.
Each of the four fluorine atoms is bonded to one selenium atom at the tip of the pyramid. The molecular geometry of SeF4 is see-saw and electron geometry is trigonal bipyramidal this is because the selenium central atom has one lone pair on equatorial position and 4. What is the bond angle of sef6 The whole structure is symmetrical in nature.
I cant draw it out on the website but if you were to do so you would see that Selenium Tetraflouride has 4 bonding pairs of elections and 1 lone pair. Tetrahedral Non-polar Dispersion See saw Polar Dipole-dipole Tetrahedral Polar Dipole-dipole See Saw Non-polar Dispersion. This gives 5 electron pairs to Se which results in trigonal bipyramidal electron pair geometry and see-saw molecular geometry.
Is it polar or non-polar. Bond dipoles do not cancel. As in your case SeF4 has S6174 34 Here the quotient is 4 and remainder is 2.
Furthermore what is the molecular geometry of sebr4. What is the most prominent intermolecular force acting on it. Of hybrid orbitals in the molecule from which you can determine the geometry of the molecule.
SeF4 Lewis Structure - How to Draw the Lewis Structure for. Of hybrid orbitals in the molecule from which you can determine the geometry of the molecule. This means that SeF4 has Trigonal Bipyramidal structure with 4 bond pairs and 1 lone pair.
Here the quotient is 4 and remainder is 2. What are the electron-pair geometry and the molecular structure of each of the following molecules or ions. What is the molecular geometry of SeF4.
Determine the molecular geometry of SeF4 a Tetrahedral b trigonal bipyramidal c t-shaped d seesaw. In both molecular arrangements the electronic geometry is octahedral with 90 angles. One may also ask is SeF4 polar or nonpolar.
Of hybrid orbitals in the molecule from which you can determine the geometry of the molecule. The molecular shape considers only the Se-Br bonds. Bond dipoles do not cancel.
The Correct Answer is. The other selenium atom is bonded to the fluorine atoms at the base of the pyramid. Axial-equatorial 90.
And all the bond angles are 90 perfect octahedral. Consider the following statements and determine which are. The way to determine the molecular geometry of SeF4 is to first draw the Lewis Dot Structure.
SeF4 has tetrahedral geometry meaning its shape is a pyramid. 6 The bond angles are. What is the correct electron geometry of SeF4.
SF4 has sp3d hybridization and is polar in nature. 5 The VSEPR notation is AX₄E. Molecules having a molecular formula of ax4e have trigonal bipyramidal molecular geometry.
Axial-axial 180. OctahedralThe molecular geometry of SeF 6 is octahedral with symmetric charge distribution on the central atomTherefore this molecule is nonpolar. Its shape in the gaseous phase is similar to that of SF 4 having a see-saw shape.
You can put these on the central Se atom. Herein is SeF4 polar or nonpolar. This means that SeF4 has Trigonal Bipyramidal structure with 4 bond pairs and 1 lone pair.
SF4 is a chemical formula for Sulfur Tetrafluoride. Furthermore What is the shape of CH molecule. 4 The hybridization that corresponds to five electron pairs is sp³d.
This means that SeF4 has Trigonal Bipyramidal structure with 4 bond pairs and 1 lone pair. How is it. SF4 is AB4U 5 domains and is trigonal bipyramidal for the electronic geometry and see-saw for molecular geometry.
Top What Is The Molecular Geometry Of Sef4 most complete. What is the molecular geometry of SeF4. As with SF₄ the shape is a see-saw.
A SF6 b PCI5 c BeH2 d CH3 93. Predict the electron pair geometry and the molecular structure of each of the following molecules or ions. Lewis Structures and Shapes of Molecules Teaching Resources imgtes.

How Many Possible Fsef Bond Angles Are Present In Sef4 Molecule
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

Consider The Molecule Sef4 A Draw The Lewis Structure B What Is The Hybridization Of Se C What Is The Electron Geometry D What Is The Molecular Geometry E What Degree Angles

Sf4 Molecular Geometry Shape Youtube
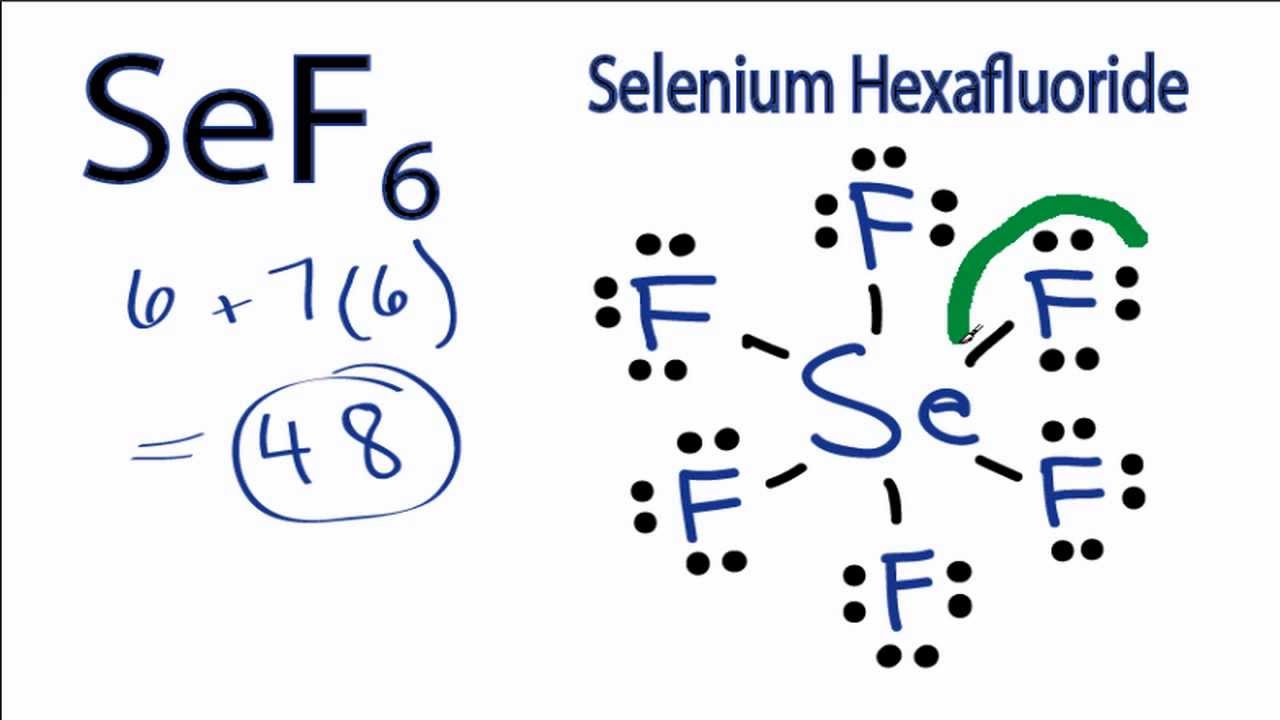
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube
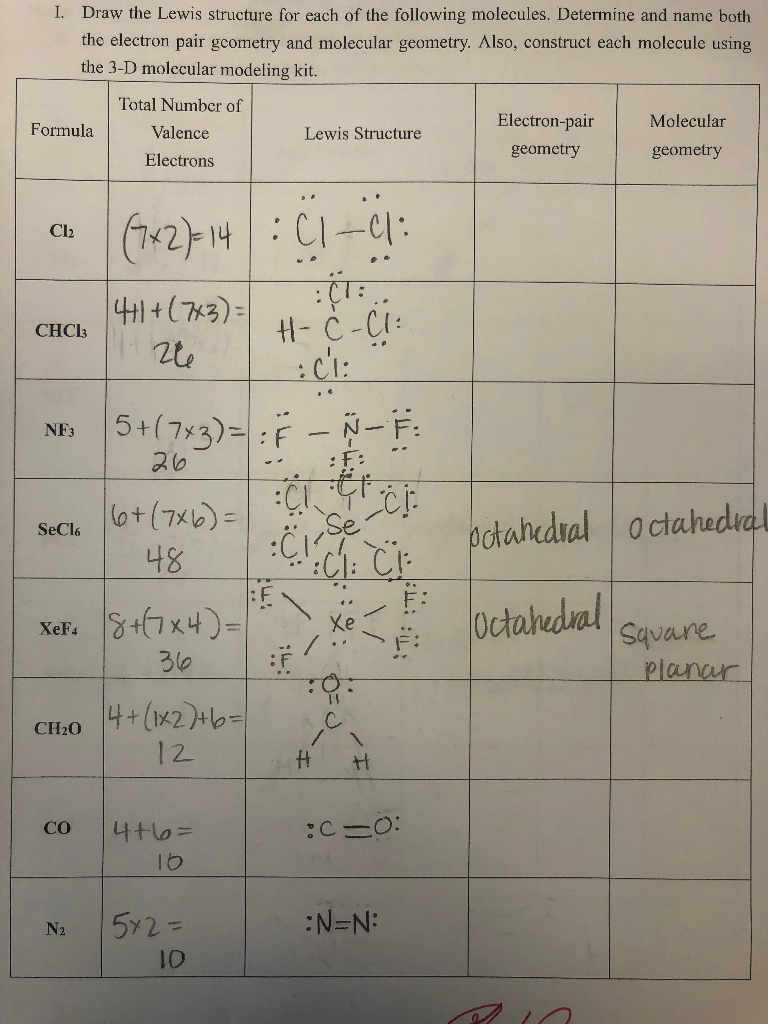
Draw The Lewis Structure For Each Of The Following Chegg Com

Solution Draw The Lewis Structure For Sef Clutch Prep
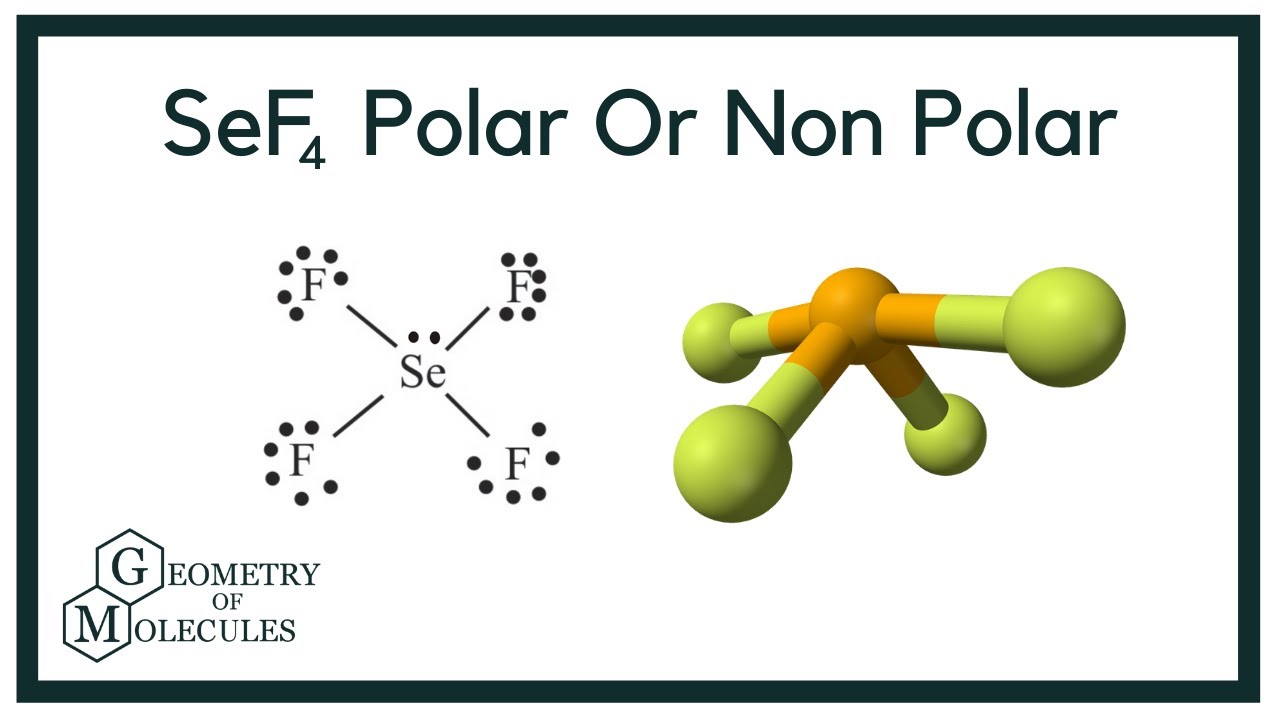
Is Sef4 Polar Or Non Polar Selenium Tetrafluoride Youtube
What Is The Molecular Geometry Of Sef4 How Is It Determined Quora

Determine The Molecular Geometry Of Sef4 Clutch Prep

Hybridization Of Sf4 Sulfur Tetrafluoride Youtube
1 What Is The Lewis Structure For Sef 4 2 What Is Its Electron Geometry 3 What Is Its Molecular Geometry 4 What Is Its Hybridization 5 How Would You Classify It In

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube
Http Www Msubillings Edu Sciencefaculty Handouts Wiles Chem 20116 Solution 20set 202 Pdf
For Sef4 Write The Lewis Structure Showing All Chegg Com
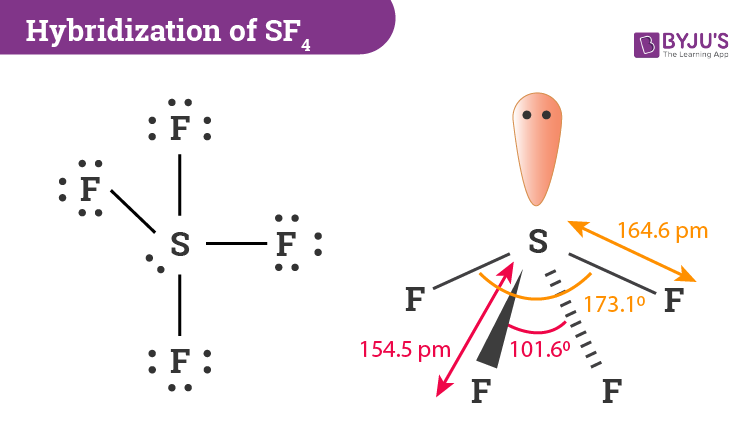
Hybridization Of Sf4 Hybridization Of S In Sulfur Tetrafluoride

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube