Xef4 Lewis Structure Hybridization
XeF4 Hybridization Hybridization of orbitals take place in the central atom Xenon. If we look at the process of synthesis of xenon difluoride heres.

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube
Now we have to determine hybridization of XeF4.

Xef4 lewis structure hybridization. XeF4 Lewis Structure Molecular Geometry Hybridization and MO Diagram. Well by drawing the lewis structure of xeo3 it becomes obvious that xe needs four hybridised orbitals. SeCl6 XeF4 IF5 AsCl51234 sp3d How Many of the atoms is correct in my structure a.
Of F atom is 4 so total valence electron becomes 7428. XeF2 Lewis Structure Molecular Geometry Hybridization and MO Diagram. Xef4 shape and xef4 bond angle If you aluminate the loan pair then it gives square planner structure.
Now 368 quotient 4remainder 4 Remainder 2422. The shape of the molecule my structure asbr5 hybridization of central atom electron pair. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride.
As a result there are four unpaired electrons in total. Valence electron of Xe 8 that of F7 here no. C hybridisation of xef4 is sp3d2 and structure is square planar.
Xe is in group 8 the noble gases. Then list the dominant IMF for each and rank in order of increasing boiling point. The central Xenon atoms orbitals are hybridized which results in the formation of new hybridized orbitals.
Vsepr valence shell electron pair repulsion theory to determine the molecular geometry eg and. Out of which two are in p-orbitals and the other two unpaired electrons are in d-orbitals. XeF2 is an abbreviation for the chemical compound Xenon Difluoride.
XeF4 Lewis Structure. Xenon has six electrons in its 5p orbitals and. Ionic or covalent Valence electrons.
The hybridization in Xenon is sp 3 d 2 because there is a migration of two electrons of p to d orbital which results in the formation of sigma bond with F. Loan pair is repulsion. But when this atom is in an excited state two electrons in the p-orbitals move to d-orbitals.
XeF4 is the chemical formula of the compound Xenon Tetrafluoride. These fluorine atoms will then be placed on either side of the central atom. One more than the 4 for sp3 hybridization the Lewis structure means.
Xe 2F2 XeF4. Xef2 Lewis Structure Polarity Hybridization and shape. Out of these compounds XeF2 is the most.
XeF4 Hybridization Circle one. XeF 4 Molecular Geometry And Bond Angles XeF 4 consists of two lone pair electrons. A CH3NH2 b CH2CIF c XeF4 Draw the Lewis structure for CS2 a What is the electron pair geometry and molecular geometry.
The Lewis structure for XeF4 has a total of 36 valence electrons. And bond angle is 90 each. For the following draw the Lewis structure 3D structure and show with arrows whether each is polar or nonpolar.
The hybridization of xef4 is sp3d2 because there is properly shifting two electrons of 5p orbitals to 5d orbitals. Adding we get 82836. What is the hybridization of the central atom GN Lewis first proposed this theory in 1916 that helps in understanding the involvement of electrons informing the structure of the chemical.
Of F atom is 4 so total valence electron becomes 7428. Adding we get 82836. Atoms number of valence electrons minus number of electrons assigned to it Part C 16 points.
Apart from XeF2 there are other Xenon compounds such as XeF4 Xenon Tetrafluoride and XeF6 Xenon Hexafluoride. XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. Now X quotient remainder2426.
This results in 4 unpaired hybridized electrons which consist of 2 in 5p and 2 in 5d orbitals. Now if we follow the VSEPR theory the net electronic repulsions has to be minimum. Now X quotient remainder2426.
This chemical compound is formed when xenon reacts with fluorine. As a result hybridization is possible. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
NF3 is pf4 polar or nonpolar H2S and AlF4- oftentimes the. Sum of attached atoms lone pairs 2. Now we have to determine hybridization of XeF4.
We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. It is a powerful fluorinating as well as an oxidizing agent. Xef2 Lewis Structure Polarity Hybridization and shape IXeF2 is a linear.
Valence electron of Xe 8 that of F7 here no. These hybridized orbitals lead to sp3d2 hybridization in XeF4. Practice - Show the calculations for the formal charge of each atom in carbon dioxide.
So it converted octahedral to square planner. Now looking on the table described above hybridization of XeF4 sp3d2. Now 368 quotient 4remainder 4 Remainder 2422.
Pf4 hybridization Total ins Generic Formula Picture Bonded Atoms Lone Pairs Molecular Shape Electron ridi -zation Bond Angles AX 5 AsF 5 AX 4E SeH 4 AX. In this process elemental fluorine supposedly oxidizes xenon under some specific conditions of temperature. Now looking on the table described above hybridization of XeF4 sp3d2.
Its chemical equation could simply be written as. Lewis Structure 3D Shape with bond angles Polar. The hybridization in Xenon is d2sp2 because there is a migration of two electrons of p to d orbital which results in the formation of sigma bond with F.
The electron structure that shows is called the Lewis Structure of XeF4. In this case one s and three p orbitals hybridise to form four sp3 hybrid orbitals.

Hybridization Of Xef4 Xenon Tetrafluorid Youtube

Theories Of Covalent Bonding Ppt Download
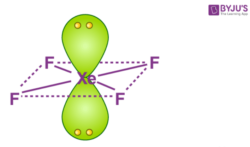
Hybridization Of Xef4 Hybridization Of Xe In Xenon Tetrafluoride
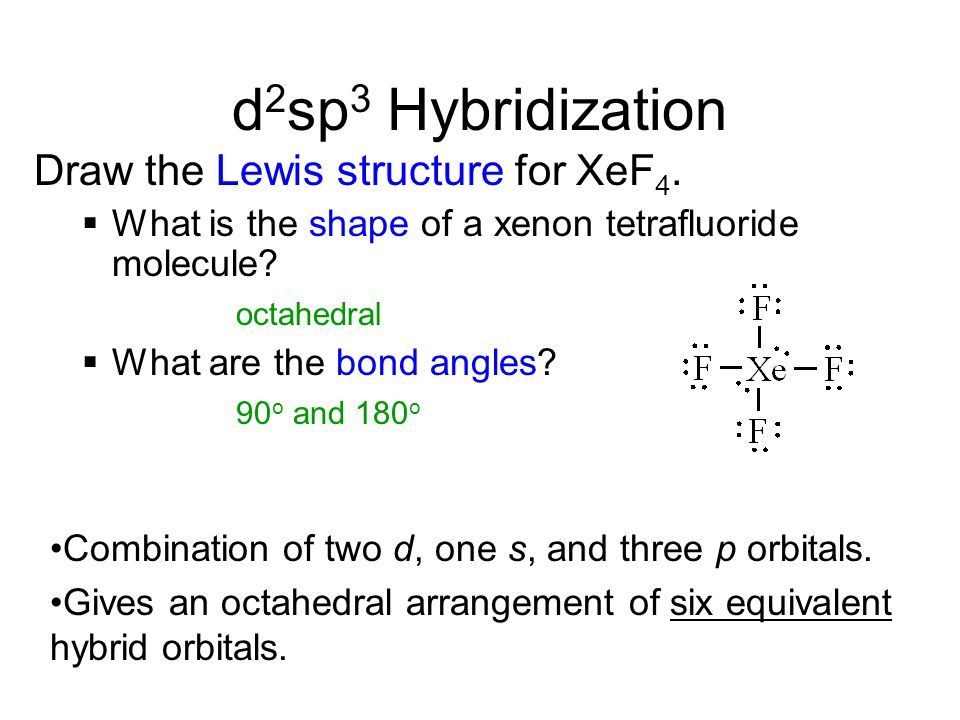
Localized E Model And Hybrid Orbitals Sigma And Pi Bonds Ppt Video Online Download
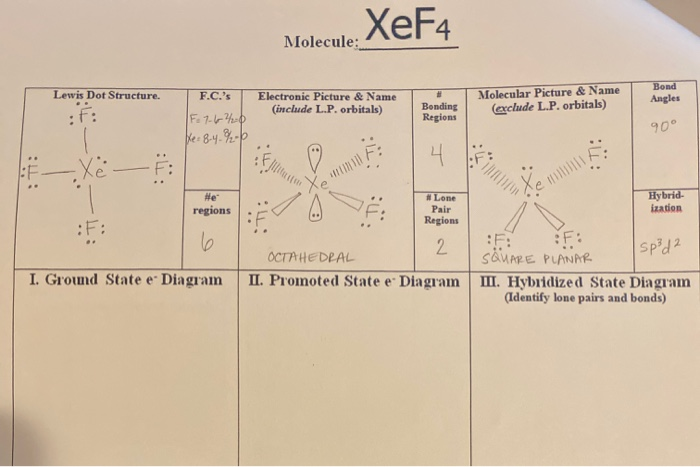
Xef4 Molecule Lewis Dot Structure F C S Electronic Chegg Com

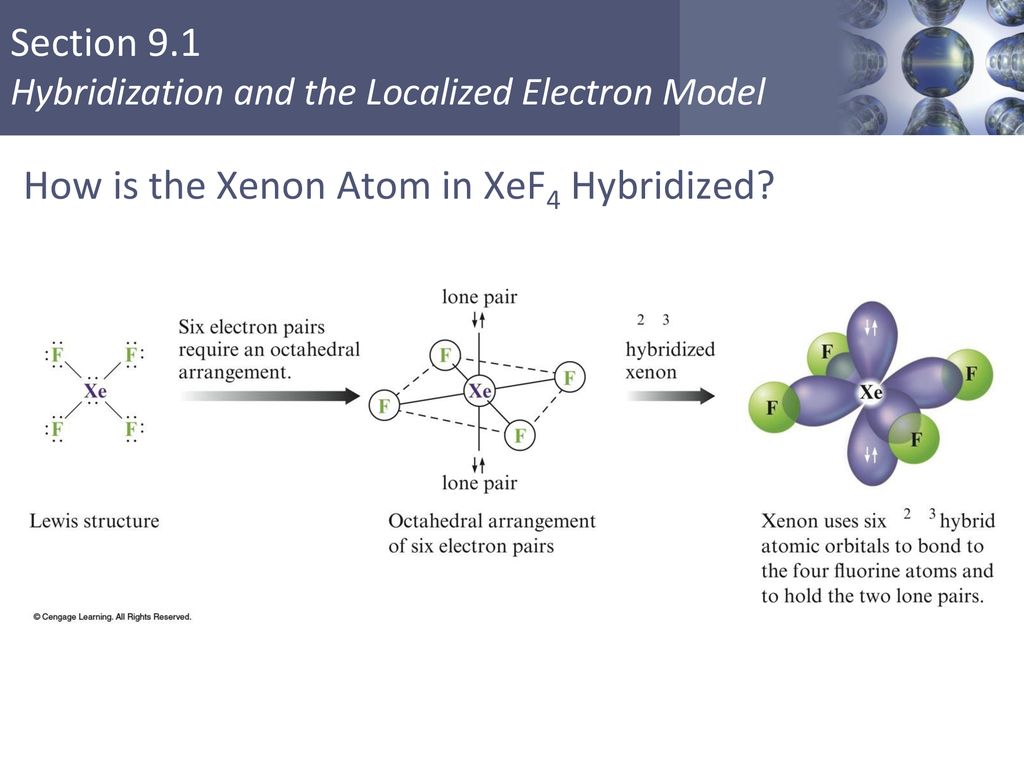
Hybridization Of Orbitals Sections 9 1 And Ppt Video Online Download
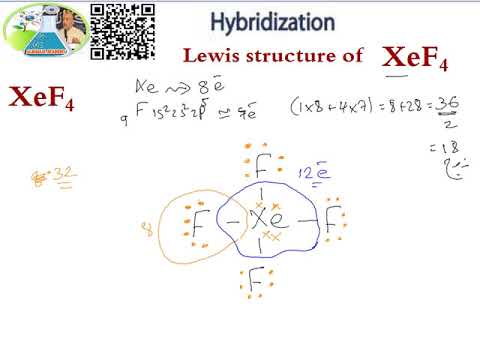
Lewis Structure Hybridization Xef4 Youtube

Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Hybridisation Of Xef4 Homeworklib
What Is The Hybridization Of Xef4 Quora

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Xef4 Lewis Structure And Molecular Geometry Youtube

Answer Determine The Electron Geometry E Clutch Prep

Hybridization Of Xef4 Xenon Tetrafluorid Youtube
What Is The Hybridization Of Xef4 Quora
Draw The Lewis Structure For Methane Ch4 Ppt Download

Chemsitry 101 Valence Bond Theory Determining Hybridization Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube