Brf3 Lewis Structure Polar Or Nonpolar
As a fluorinating reagent it is an interhalogen chemical with bromine and fluorine. The polarity across the Br-F bond is generated by the difference in electronegativity of bromine and fluorine atoms with bromine as the positive pole and fluorine as the negative pole.
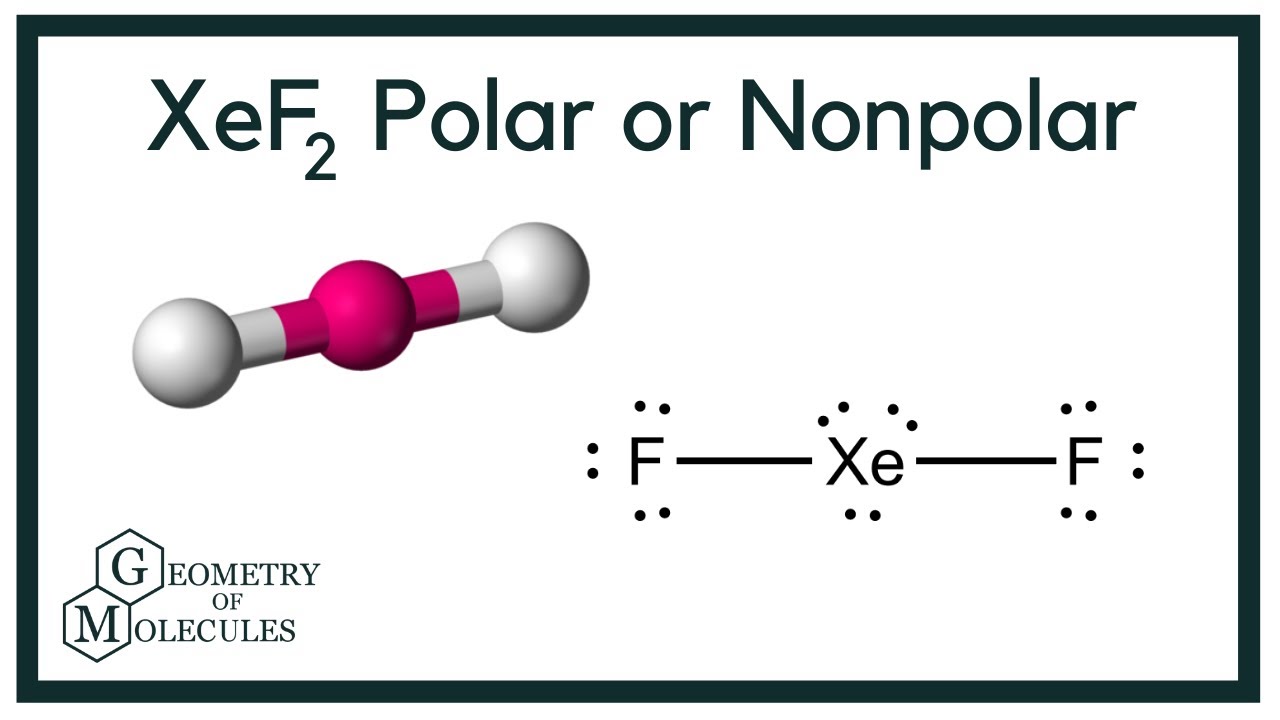
Hybridization Of Brf3 Bromine Trifluoride Youtube
Which has the highest polarity out of.

Brf3 lewis structure polar or nonpolar. We investigate that further with geometry Lewis structure and other polarity related properties. Bromine pentafluoride BrF5 lewis dot structure molecular geometry polar or non-polar bond angle. Draw the Lewis structure for BrF3 in the window below.
And the distribution of charge on its atoms is non-uniform and the molecule turns out to polar in nature. Lewis structure of some other related post in this blog. It is used as a propellent of rockets and fluorinating chemicals.
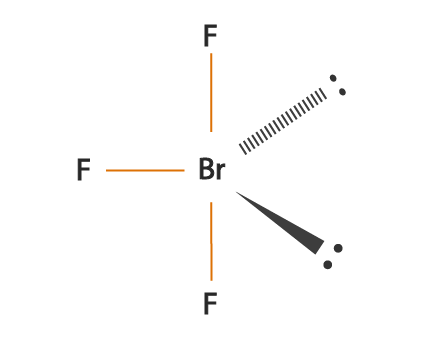
Drawing BrF3 Lewis Structure is very easy to by using the following method. Is BrF3 Polar or Nonpolar. BrF3 bromine trifluoride is a polar molecule because of the presence of two lone pairs on bromine atom due to which the shape of the molecule is distorted or bent.
What is the lewis structure of PF5. This problem has been solved. BrF3 is a polar molecule.
Bromine trifluoride chemical formula is BrF3. However to determine if N2O is polar we consider the molecular geometry. See more detail by clicking on it H2O BeCl2 SF4 NH3 XeF4 BF3 BrF3 BrF5 and CH2Cl2 molecules.
Therefore we have a bent T-shape for the BrF3 molecule. SO3 molecule is a mixture of one sulfur and three oxygen atoms. Three fluorine atoms surround one bromine atom in bromine trifluoride.
Here in this post we described step. BrF3 molecule is polar. If you look at the Lewis structure for N2O it appears to be a symmetrical molecule.
Lewis structure is one of the oldest methods to. As there is a high difference between the electronegativity of Br and F atoms. In this post we discussed the method to construct the CH3I Lewis structure.
Second place the valence electron on the iodine and hydrogen atoms. When the difference in electronegativity between the two atoms is less than 05 it is majority nonpolar. Because of its highly symmetric form BF3 Boron Trifluoride is Non-Polar.
BF3 is nonpolar. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero. Hybridization Of SO3 molecule.
Learn to determine if XeCl4 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look a. Now we will analyze various factors which are responsible for polarity or nonpolarity of the BF3 molecule. The BrF3 molecule is considered a non-polar molecule.
Is BrF3 Polar Or Nonpolar. The formal charge on central sulfur atom of SO3 Lewis structure is zero. Bromine pentafluoride has the chemical formula BrF5 and is a pale yellow liquid.
Draw The Lewis Structure For BrF3 In The Window Below And Then Answer The Questions That Follow. Learn to determine if BF3 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look and. In this blog you can see more about the polarity of CH2Cl2 CHCl3 BF3 and BeCl2.
A step-by-step explanation of how to draw the BrF3 Lewis Dot Structure Boron trifluoride For the BrF3 structure use the periodic table to find the total n. It has a Trigonal Planar geometry that cancels out the dipole moments of the three B-F bonds resulting in a compound with a Dipole Moment of 0 value Zero. The charges are distributed non-uniformly across the entire molecule.
I hope this article made sense to you and helped you to understand BF3 Lewis Structure Molecular Geometry Hybridization and Polarity. BrF3 is a polar molecule due to the presence of two pairs of lone pair electrons. First the valence electrons are placed around the carbon atom.
What is the lewis dot structure for I3-. Ill tell you the polar or nonpolar list below. Bromine trifluoride is an interhalogen compound.
Learn to determine if C2H4 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and then us. Hence the total number of valence electrons for BrF3 is 28.

Is Brf3 Polar Or Nonpolar Techiescientist

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Brf3 Lewis Structure Bromine Trifluoride In 2021 Lewis Chemical Formula Dots
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Brf3 Polar Or Nonpolar Bromine Trifluoride Youtube
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
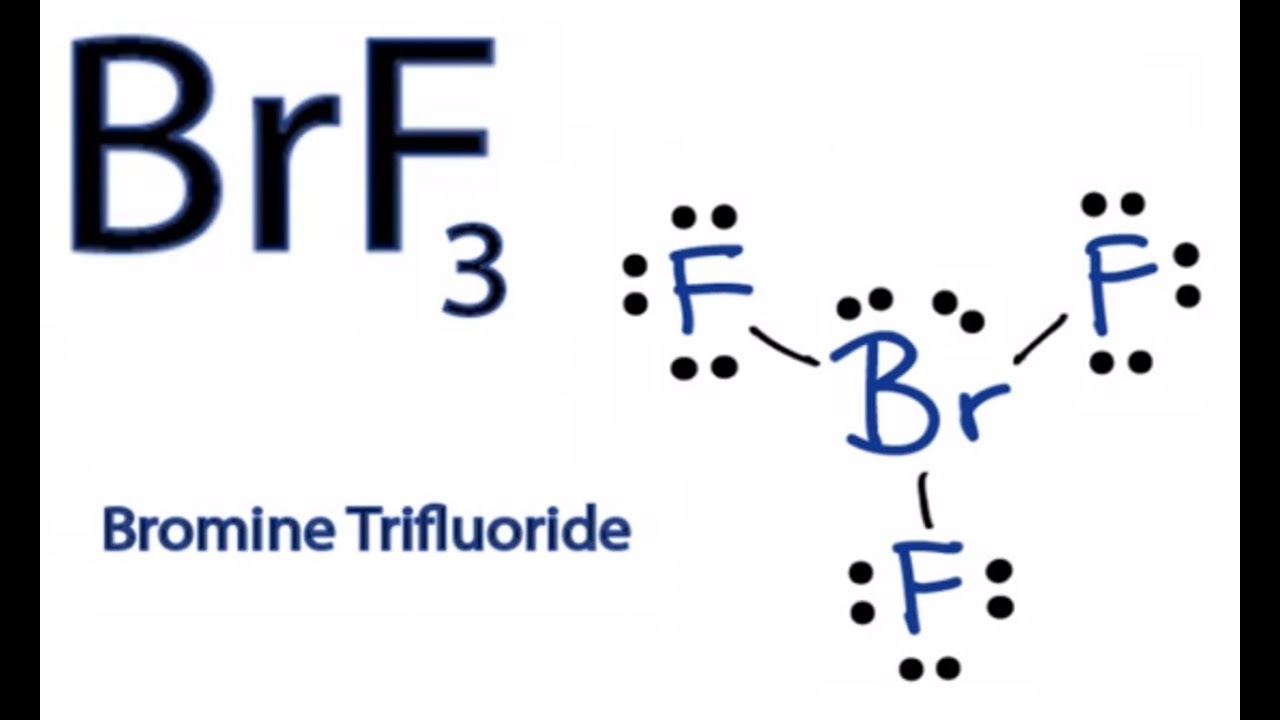
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube

Brf3 Polar Or Nonpolar Bromine Trifluoride Youtube

Is Brf3 Polar Or Nonpolar Techiescientist
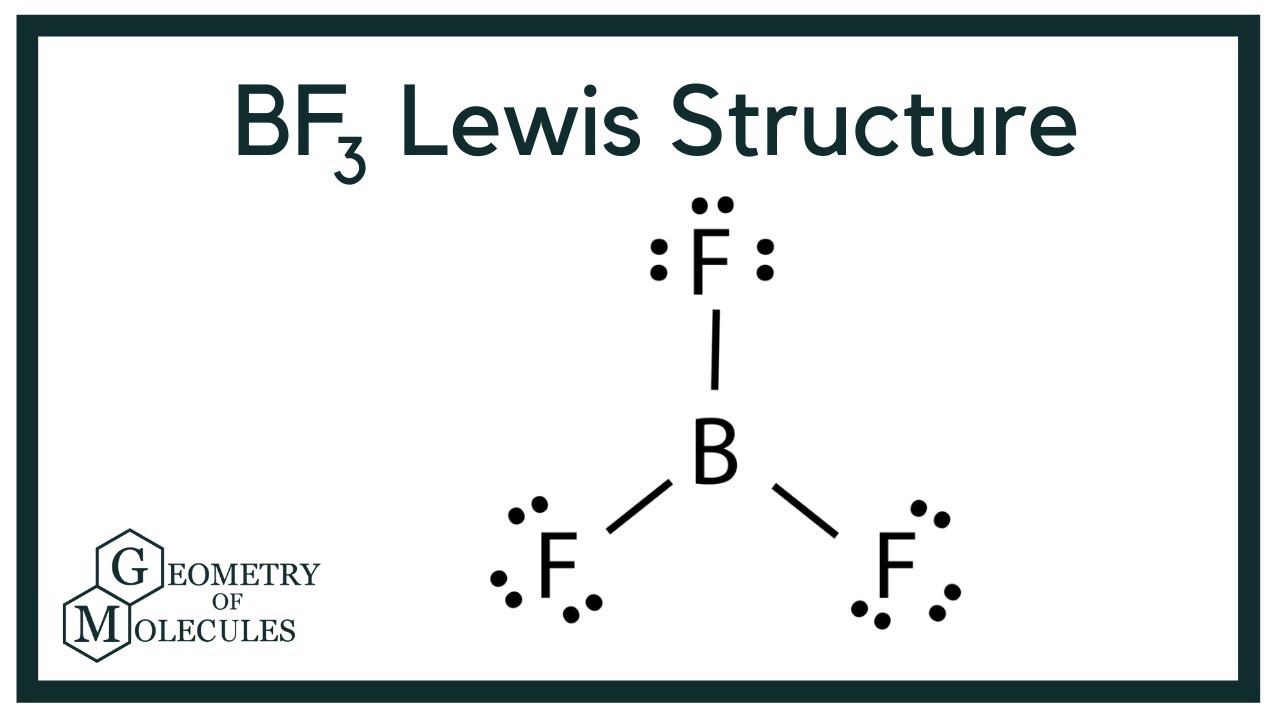
Bf3 Lewis Structure Boron Trifluoride Youtube
Brf3 Bromine Trifluoride Molecular Geometry Bond Angles Youtube

5 Draw The Most Appropriate Lewis Structure S For Brf3 Wh Clutch Prep

Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Brf3 Polar Or Nonpolar Bromine Trifluoride Youtube

Best Overview On Bf3 Polar Or Nonpolar 1 Science Education And Tutorials

Brf3 Polar Or Nonpolar Bromine Trifluoride Youtube