Brf5 Lewis Structure Hybridization
The steric number of the central Br atom is 6 five sigma bonds and a lone pair of electrons are present on the central Br atom so the hybridization of Br in eqBrF_5 eq is speq3 eqd. What is the hybridization of the central atom in BrF5.
Brf5 El Brillill Molecule Lewis Dot Structure Chegg Com
We first draw the Lewis structure of bromine pentafluoride eqrm BrF_5 eq.
Brf5 lewis structure hybridization. Is BrF5 a dipole moment. To determine the hybridization we take a look at the Lewis structure of the BrF 5 molecule. For human beings bromine pentafluoride is highly toxic and must not be inhaled at any cost as it is.
A sp3d2 B sp3d C sp3 D sp2 E sp. Is XeF2 a Lewis structure. The molecular formula for nitromethane is CH3NO2 a.
Draw a valid Lewis structure for this molecule. BrF5 Lewis Structure Molecular Geometry Hybridization and Polarity. The central atom of the AlCl3 lewis structure is exceptional to the octet rule as it holds stability with only 6 electrons.
Label one of the oxygens on your structure a and the other b. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds. What is the hybridization on the Br atom.
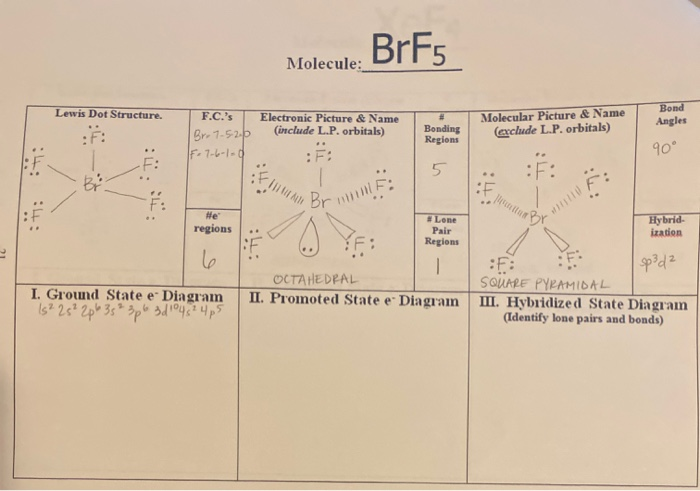
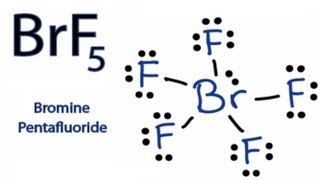
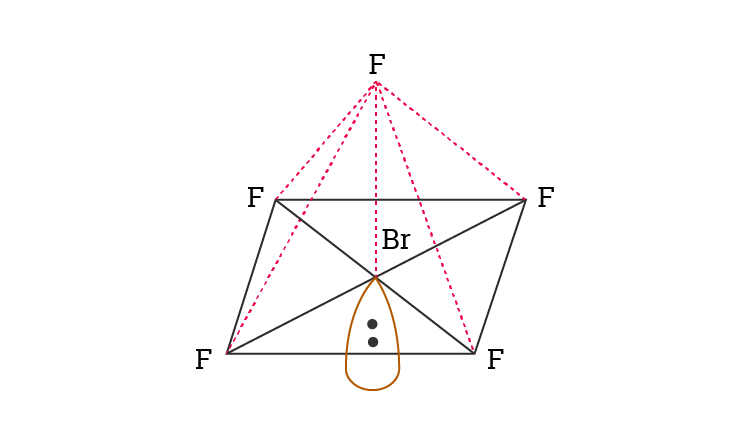
The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral. BrF5 or bromine pentafluoride has a square pyramidal structure as in the first figure. See full answer below.
There are seven valence electrons in the central atom iodine and 5 electrons form 5 sigma bonds with the atoms F. Next draw the 3-dimensional structure for BrF5 using VSEPR rules. The molecule will consist of one lone pair.
The hybridization of Bromine in the BrF5 is SP³d². 23 Give the hybridization for the Br in BrF5. With bromine and each of the 5 fluorine atoms having 7 valence.
Having a straw ie colorless to yellow appearance this chemical compound has several applications but also comes with a number of limitations and. QUESTION 22 Draw the Lewis structure for BrF4. In this tutorial we will draw the lewis dot structure of AlCl3 with simple steps and all possible explanations.
Indicate the hybridization of the. Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs. The hybridization of BrF5 is Sp³d².
During the hybridization one 4s three 4p and two 4d orbitals take part in the process giving rise to sp 3 d 2 hybrid orbitals. O 0 l o l b. Five valence electrons of bromine will be used to form sigma bonds with 5 F atoms.
What is the hybridization on the Br atom. There is also a lone pair attached to the Bromine atom. The molecule has a central bromine atom that is surrounded by five fluorides and a lone pair of electrons.
In the liquid state it is a colorless fuming compound having a pungent odor. Bromine Pentafluoride comprises 5 Fluorine atoms all pulled together by the central Bromine atom. AlCl3 lewis structure contains three bonded pairs and a total of 9 lone pairs.
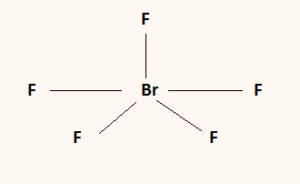
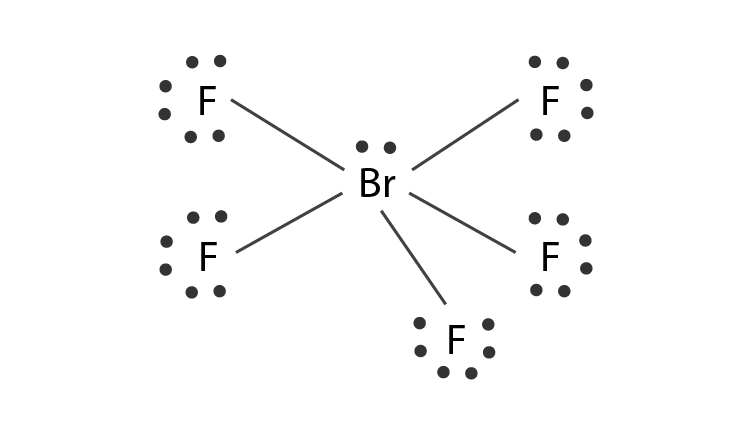
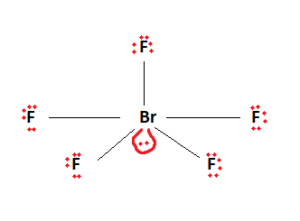
BrF3 Lewis Structure Molecular Geometry Hybridization and MO Diagram BrF3 known as Bromine Trifluoride is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. BrF5 lewis dot structure has 10 sharing electrons and 32 non-sharing electrons. The total valence electron available for drawing the BrF5 lewis structure is 42.
The remaining 2 electrons form 1 lone pair. The hybridization of BrF5 is determined by the number of sigma bonds and lone pairs. QUESTION 22 Draw the Lewis structure for BrF4.
Bromine pentafluoride is polar in nature. In BrF 5 one 4s three 4p and two 4d orbitals take part in hybridization. A sp3d2 B sp3d C sp3 D sp2 E sp.
A video explanation of how to draw the Lewis Dot Structure for Bromine Pentafluoride along with information about the compound including Formal Charges Pol. There are a total of 22 valence electrons in the Lewis structure for XeF2. Bromine Pentafluoride or BrF5 is a fluoride of bromine and an interhalogen compound which means it is made up of only halogen atoms.
The electron geometry is octahedral and the hybridization is sp3d2. It is used as a propellent of rockets and fluorinating chemicals. Valince bonding e a8 non-bonding e.
SOLUTION a The Lewis structure of the SnCl3 ion looks like this. YOU MUST SHOW THE FINAL LEWIS STRUCTURE ON YOUR CALCS SHEET A sp3d2 B sp3d C sp3 D sp2 E sp 24 Determine the freezing point depression AT of a solution that contains 307 g glycerin C3Hg03 molar mass 9209 gmol in 376 g of water. The polarity is best found by first drawing the Lewis dot structure for BrF5.
The lone pairs are around iodine and sigma bonds formed between the F and Br. But on the other hand BrF5 does have a dipole moment due to the asymmetric structure as shown earlier in the figures. It can be observed from the BrF 5 Lewis structure that there are five Fluorine atoms surrounding the central Bromine atom.
Important Points To Remember. As a fluorinating reagent it is an interhalogen chemical with bromine and fluorine. Bromine pentafluoride BrF5 lewis dot structure molecular geometry polar or non-polar bond angle Bromine pentafluoride has the chemical formula BrF5 and is a pale yellow liquid.

How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
Bromine Pentafluoride Brf5 Lewis Dot Structure Youtube
What Type Of Hybridized Orbital Is Used By The Central Atom Of Brf5 Quora
What Type Of Hybridized Orbital Is Used By The Central Atom Of Brf5 Quora

Brf5 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Brf5 Bromine Pentafluoride Molecular Geometry Bond Angles Youtube
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride Youtube

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Hybridization Of Brf5 Hybridization Of Br Bromine In Brf5
What Type Of Hybridized Orbital Is Used By The Central Atom Of Brf5 Quora

Brf5 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
What Is The Hybridisation Of Brf5 Quora

Brf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
