C2h4cl Lewis Structure
The Lewis Structure for Li. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
Https Nanopdf Com Download Organic Chemistry 101 Problems 1 Answers Pdf
It is a colorless flammable gas or refrigerated liquid with a faintly sweet odor.

C2h4cl lewis structure. 42 What is the Lewis structure for chloroethylene C2H3Cl. Chloroacetic acid structure C 2 H 3 O 2 Cl. The second one is 11-dichlororethane.
The Lewis Structure or Lewis Dot Diagram shows the bonding between atoms of a molecule and any electrons that may exist. Dichloroethane 12-dichloroethane 107-06-2 ethylene dichloride ClCH2CH2Cl. View this answer View this answer View this answer done loading.
I think its similar to the Lewis structure for PCl5. Calculate the total number of valence electrons present. It is combustible and corrosive.
It is toxic when ingested inhaled or absorbed through skin. Use information from step 4 and 5 to draw the lewis structure. Alternatively a dot method can be used to draw the lewis structure.
This problem has been solved. C2H4ClF 1-Chloro-2-fluoroethane Quadrupole constants in 1-chloro-2-fluoroethane Molecular structure of 1-chloro-2-fluoroethane Reactions in anhydrous hydrogen fluoride Communication 1. Thus both are valid Lewis structures and both molecules exist.
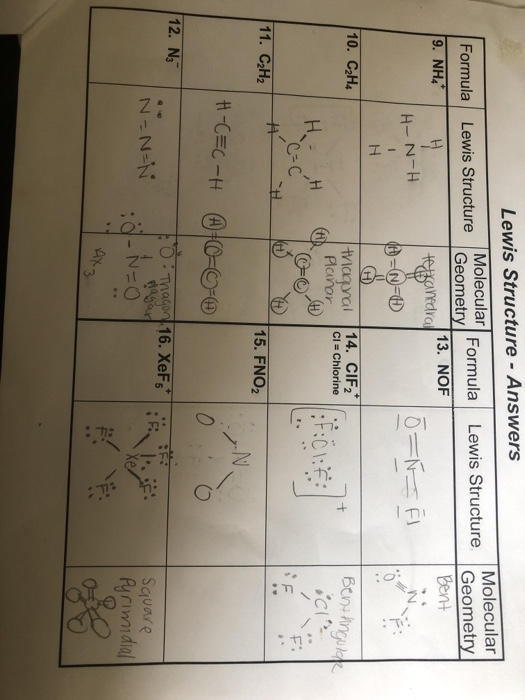
In the Lewis structure for C 2 H 2 Cl 2 there are a total of 20 valence electrons. The first one is 12-dichlororethane. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them.
In a double bond two pairs of valence electrons are shared for a total of four valence electrons. It dissolves and sinks in water. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Electron Dot Structure for ethane C2H4. 167 and an odour resembling that of chloroform it is used as a solvent for oil and grease in metal cleaning and in the separation of coal from impurities. Lewis structures of acetaldehydeethylene oxide and vinyl alcohol.
Were being asked to draw the 3 Lewis structures for C 2 H 2 Cl 2 and indicate whether each is non-polar or polar. To do so we first need to do the following steps. So if you type that structure into Google you should receive the Lewis structure.
Find octet e- for each atom and add them together. The two Lewis structures for the given molecular formula are shown below. Calculate the total valence electrons in the molecule.
The key to understanding how to distribute the valence electrons is to. C 2 H 3 O 2 Cl Uses Chloroacetic acid. It causes thermal burns when transported in its molten liquid form.
Find valence e- in all atoms. This means that the carbon atoms share 4 electrons. View a sample solution.
Step 3 of 3. Chlorethylenoxide C2H4Cl- CID 19069940 - structure chemical names physical and chemical properties classification patents literature biological activities. Remember that Hydrogen H atoms always go on the outside of a Lewis Structure.
Lewis dot structure of C 2 H 4. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Cl 2 Lewis structure. Who are the experts.
Determine the central atom in this molecule. See the answer See the answer See the answer done loading. Note that Hydrogen only needs two valence electrons to have a full outer shell.
Structural Isomers are 2. In its solid form it is a colorless or light-brown crystalline solid. HH H- C- C- Cl Oc H-CC- O d.
12-Dichloroethane-d4 C2H4Cl2 CID 12197860 - structure chemical names physical and chemical properties classification patents literature biological. H H Cl--C--C--Cl H H. Structure properties spectra suppliers and links for.
Step 2 of 3. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule. Chloroethane commonly known as ethyl chloride is a chemical compound with chemical formula CH 3 CH 2 Cl once widely used in producing tetraethyllead a gasoline additive.
H HI- H-CC- b. Chapter 1 Problem 9P is solved. Show transcribed image text Expert Answer.
Conjugated halogenation of olefins Testing the atomic orbital graph as a basis for QSPR modeling of the boiling points of haloalkanes null. Draw the Lewis structure for the molecule.

C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube
Lewis Structure Answers Molecular Wis Structure Chegg Com

Organic Chemistry 101 Problems 1 Answers
How Do You Know The Hybrid Orbitals Of A Compound For Example What Are The Hybrid Orbitals Of Ch 2cl 2 C 2h 4 And C 2h 2 Socratic

C2h4 Molecular Geometry Shape And Bond Angles Youtube

Write The Electron Dot Structure Of Ethene Molecule C2h4

How To Balance C2h4 Cl2 C2h4cl2 Ethene Chlorine Gas Youtube
Illustrated Glossary Of Organic Chemistry Kekule Structure
Https Nanopdf Com Download Organic Chemistry 101 Problems 1 Answers Pdf

How To Name Ch3och2ch3 Youtube
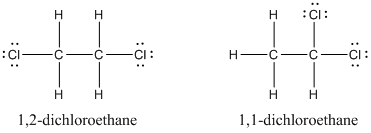
Solved Draw Lewis Structures For Each Molecular Formulaa C2h4cl2 Chegg Com
Illustrated Glossary Of Organic Chemistry Kekule Structure

Write The Electron Dot Structure Of Ethene Molecule C2h4
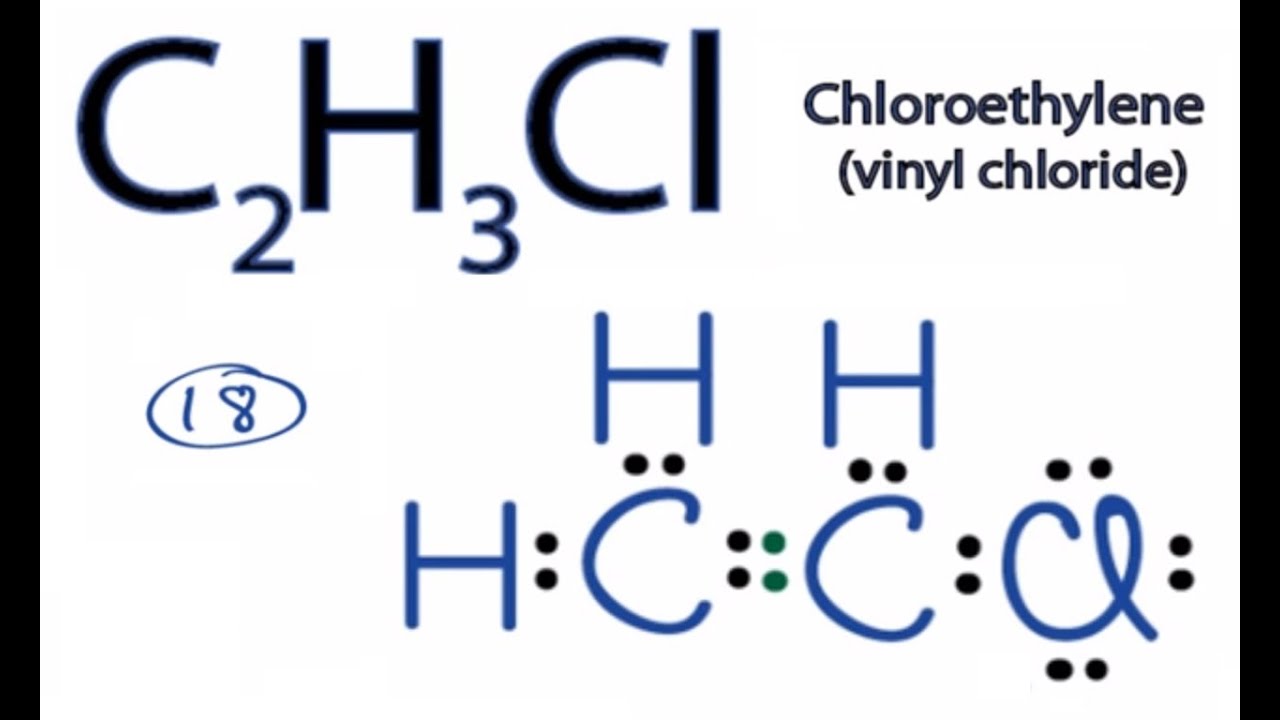
C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

Is C2h4 Polar Or Non Polar Ethylene Youtube

C2h4 Molecular Geometry Shape And Bond Angles Youtube

How To Balance C2h4 Cl2 C2h4cl2 Ethene Chlorine Gas Youtube
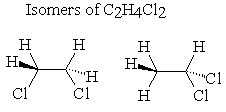
How Many Isomers Does C2h4cl2 Have Socratic