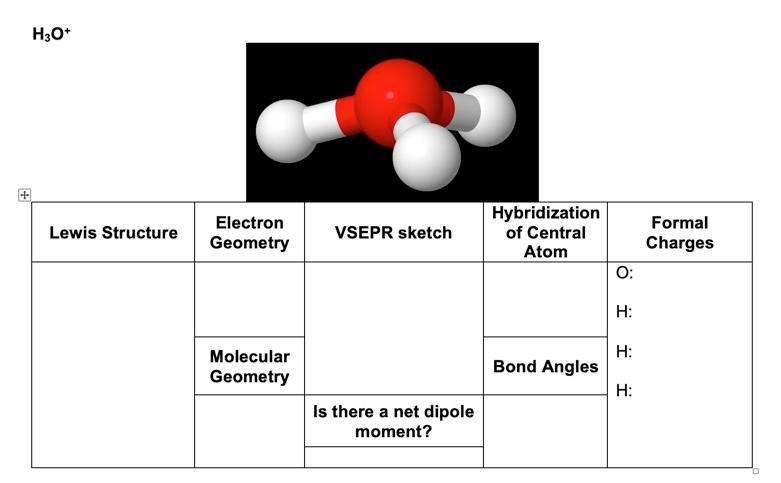
H3o+ Lewis Structure Bond Angles
Bond angles between 109-120. Order0764___ between O1 and H4.

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
A video explanation of how to draw the lewis dot structure for hydronium ion along with information about compound including formal charges polarit.

H3o+ lewis structure bond angles. Indicate the molecule with the greater bond angles. Carbon in center AB3 trigonal planar bond angles 120. Lets try to draw the lewis structure of H3O.
For the molecule H3O give the following. Now the important point is not to forget about the sign. Postby Reva Kakaria 1J Mon Nov 19 2018 927 pm Since H3O has a lone pair and three bonds I think the bond angles would be.
Order0764___ Top of page. The model also states that the molecular geometry of the compound is trigonal planar with each orbital equidistant at 120 degrees bond angle shaped on a planar region. Ordering Bond Angles Exercise EXERCISE 62.
It is a conjugate acid of a water. A quick explanation of the molecular geometry of H3O the Hydronium ion including a description of the H3O bond anglesLooking at the H3O Lewis structure. Consists of molecules spheres made of atoms arranged in hexagonspentagons.
Consider the Lewis structures of CF 4 SO 3 SO 2 NF 3 and OF 2 which are given below. Angle1132 deg___ for H4-O1-H2. The molecular geometry and the shape of the water molecule are.
For H3Oprovide a Lewis structure predicted VSEPR molecular geometry bond angle and indicate whether the compound is polar non polar or polyatomic ion. Draw the Lewis structure for eqH_3O eq. Lewis Structure include normal wedge and dotted lines when necessary Number of valence electrons Number of bonded atoms on central atom Number of lone pairs on central atom Central atom steric number Bonded-atom lone-pair arrangement BALPA Bond angles Hybridization Number of sigma and pi bonds Molecular shape Polarity and Bond.
First of all we need to calculate the total number of valence electrons present in hydronium ion. Thus all bond angles around atoms with lone pairs are preceded by a. Sign indicates losing an electron from the total valence electrons.
Molecule F BrNS umber of valence Lewis structure Hybridization 18亡 Number of σ and π electrons n. Also go over hybridization shape and bond angle. Hydrogen 1 3Hydrogen 3 Oxygen 6 Total 9.
H3O Lewis Structure. Note only lone pairs around the central atom are shown. For the H3O Lewis structure we first count the valence electrons for.
Number of bonded atoms on central atom Molecular shape Number of lone pairs on central atom Bonded-atom lone- pair arrangement BALPA Polarity Central atom steric number Bond angles Bond order N-Br. Covalent bonding 23 structures with. For trigonal pyramidal geometry the bond angle is slightly less than 1095 degrees around 107 degrees.
There are two lone pairs on the Oxygen atom as it doesnt participate in forming bonds. A step-by-step explanation of how to draw the H3O Lewis Structure Hydronium Ion. I quickly take you through how to draw the lewis structure of hydronium ion h3o.
Order0764___ between O1 and H3. Sp 2 and sp 3. Oxonium is an oxygen hydride and an onium cation.
The formula AXn N says that A is the central atom X is the atom attached to the central atom n is the number of atoms bonded and N is the number of nonbonding electron pairs. Oxygen on right AB2E2 bent bond angle. Sp 2 and Diamond.
Best Lewis Structure The Lewis structure that is closest to your structure is determined. We will indicate that the bond angle deviates from the predicted value with a in front of the angle. The resulting molecular shape is bent with an H-O-H angle of 1045Structure properties spectra suppliers and links for.
Between O1 and H2. Layered structure two-dimensional structure planar. Molecule G Lewis structure Hybridization PFs Number of σ and π bonds Number of.
Hydrogen iodide Hydriodic acid 10034-85-2 14362-44-8It is trigonal pyramidal and sp3 hybridized. For molecules or ions with an expanded octet on the center atom lone pair repulsion will also decrease the bond angle s except in the two. The oxygen atom in the H2O molecule has sp3 hybridization and the bond angle of H-O-H is 1045.
Angle1132 deg___ Top of page. Once several lines had been identified in the laboratory the first interstellar detection of H3O was made by two groups almost simultaneously in 1986. The Lewis structure of CH_3- is The carbanion has three bonding pairs and one lone pair.
For this molecule determine the molecular geometry electron domain geometry bond angles and hybridization about the central atom.
Http Www Simonedamiano Com 043 Structures And Proper Pdf
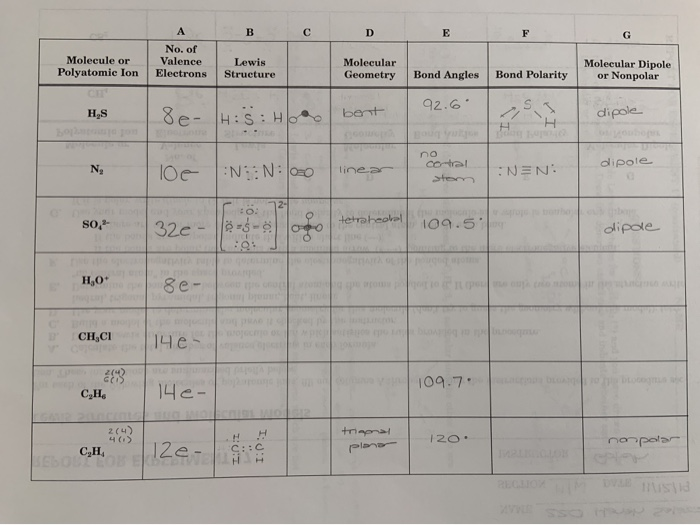
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com
What Is The Structure Of H3o Quora
How To Draw Lewis Structure For H3o Drawing Easy
H20 Lewis Structure Electron Geometry Vsepr Sketch Chegg Com

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
What Is The Structure Of H3o Quora

What Is The Shape Of H3o Ion Quora

How To Draw Lewis Structure For H3o Drawing Easy

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
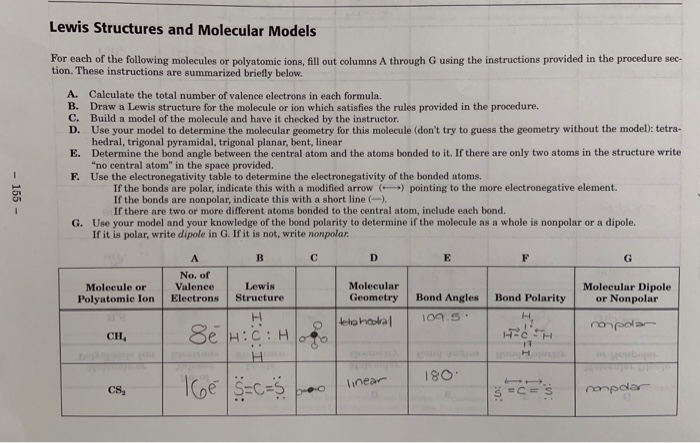
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com

H3o Molecular Geometry Shape And Bond Angles Youtube
What Is The Structure Of H3o Quora
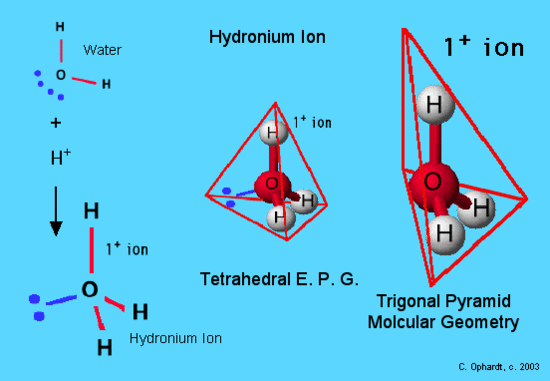
Trigonal Pyramidal Molecular Geometry Chemistry Libretexts

What Is The Shape Of H3o Ion Quora
What Is The Shape Of H3o Ion Quora

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

What Determines Molecular Shape Bond Angles Angle Formed Between Two Adjacent Bonds On The Same Atom E G Ccl 4 Chapter 9 Molecular Geometry And Bonding Ppt Download