H3o+ Number Of Valence Electrons
Lewis dot structure of h3o hydronium ion. Total valence electrons given by nitrogen atom 5.

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
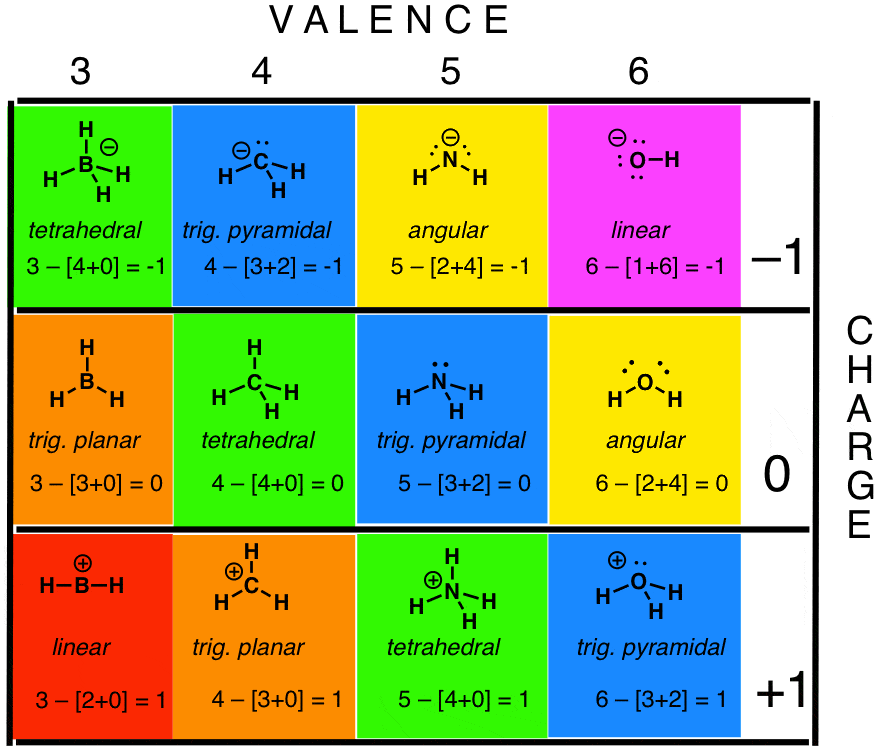
Secondly we need to resolve a central atom which is typically the atom with probably the most available sites for bonding.
H3o+ number of valence electrons. There are three oxygen atoms in NO 3- Therefore. H3O 1 total number of valence electrons2 VSEPR formula3 electron pair geometry4 molecular geometry5 bond angle6 polar or non-polar7 total number of lone pairs. A NO2 b NH4 c.
Note that the sign in the Lewis structure for H3O means that we have lost a valence electron. Elements in a high oxidation state can have a valence higher than four. Solution for H3O Valence electrons.
It is to use this distribution of electrons to predict the shape of the molecule. It is also named the Gillespie-Nyholm theory after its two main developers Ronald Gillespie and Ronald Nyholm. Total valence electrons given by oxygen atoms 6 3 18.
For neutral atoms the number of valence electrons is equal to the atoms main group number. Thus by the formula V 6 3 hydrogen atoms are bonded to oxygen so the number of the monovalent atoms M 3. Once we include nonbonding electrons that is no longer true.
Thus the total valence electron is 8 now. The charge in the molecule indicates that one of the atoms lacks 1 valence electron in this case oxygen. What determines the number of valence electrons.
The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel. Oxygen is in group 6 and has 6 valence electrons. In oxygen atom there are six electrons in its valence shell.
For example in perchlorates chlorine has seven valence bonds. Next we wish to draw a skeletal construction of H3O with unmarried bonds best. Calculate the polarity for H3O.
There are 8 valence electrons for the H3O Lewis structure. Our goal however isnt predicting the distribution of valence electrons. The main group number for an element can be found from its column on the periodic table.
What is the total number of valence electrons in each of the following. H3O is an important compound. Valence shell electron pair repulsion theory or VSEPR theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms.
Identify the molecular shape for H3O. The valence electrons on the central atom in both NH 3 and H 2 O should be distributed toward the corners of a tetrahedron as shown in the figure below. Due to -1 charge another electrons is added.
In this case we have oxygen atom as central atom so only oxygen will undergo hybridization. Solution for i State the number of valence electrons in each compound below. Write the number of valence electrons for H3O.
20 e total valence electrons 1 atom of S6 valence e 2 atoms of Br7 valence e20 e 17 e total valence electrons 1 atom of N5 valence e 2 atoms of O6 valence e17 e. Until now the two have been the same. Therefore we only have 8 valence electrons for the h3o lewis structure.
In this case Oxygen is the central atom. Ruthenium in the 8 oxidation state in ruthenium tetroxide has eight valence bonds. H3O 1 total number of valence electrons2 VSEPR formula3 electron pair geometry4 molecular geometry5 bond angle6 polar or non-polar7 total number of lone pairs.
And identify if H3O is nonpolar or polar. Now lets find the hybridization of H3O using this formula In hydronium ion the central atom is oxygen and it has 6 valence electrons. For example carbon is in group 4 and has 4 valence electrons.
Draw the lewis structure for H3O. Therefore we only have 8 valence electrons for the H3O Lewis structure. H3O is tetrahedral because it has 8 total valence electrons so there must be a bond between each of the 3 H atoms and the O leaving a lone pair on the O.
And what is the angle bond for H3O. The electrons that participate in bond formation are. Ii Draw Lewis structures for each of the following compounds.
So in keeping with the vsepr chart h3o has trigonal pyramid as its molecular form and tetrahedral as its electron geometry. Due to -1 charge received electrons to valence electrons 1. Atomic number of Hydrogen is 1 it means it has 1 electron and Atomic number of oxygen is 8 it means it has 8 electrons.
The electronic configuration of Hydrogen will be - 1 and that of Oxygen will be - 26. Hence the total number of valence electrons is 8.

How Many Valence Electrons Are In Potassium Quora

How To Determine The Number Of Valence Electrons In An Atom Using The Periodic Table Quora
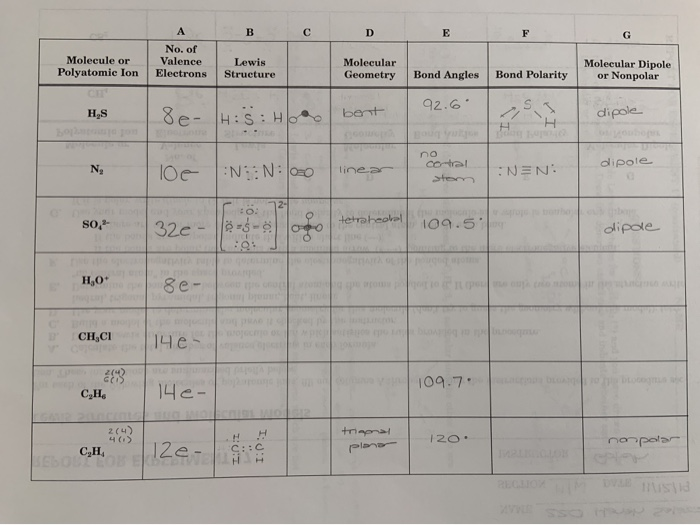
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube

Electron Dot Structure Of Hydronium Ion Brainly In
How Many Valence Electrons Are In Potassium Quora

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

Calculating Nh3 Formal Charges Calculating Formal Charges For Nh3 Ammonia Youtube
How To Calculate Formal Charge

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
How To Calculate The Formal Charges For H3o Hydronium Ion Youtube

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
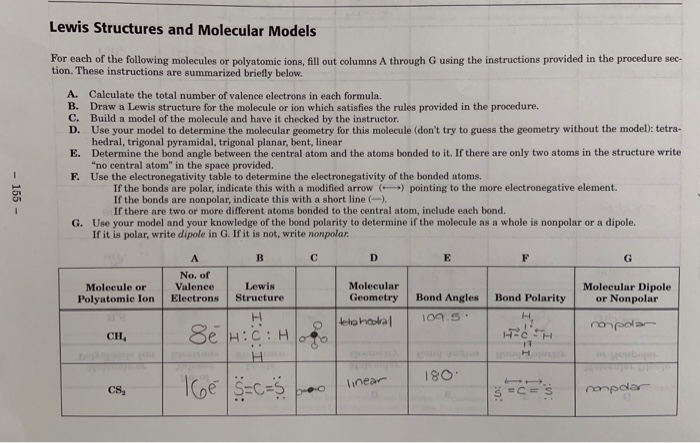
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com

What Is The Shape Of H3o Ion Quora

9 26 06 Reading 8 6 Bond Length P Exceptions P Ppt Video Online Download
What Is The Bond Angel Of H3o Quora
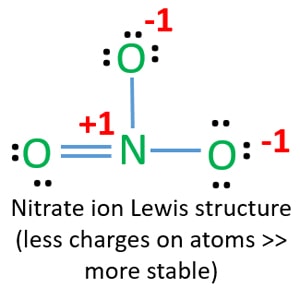
Lewis Structure Of No3 Nitrate Ion

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

H3o Molecular Geometry Shape And Bond Angles Youtube