How Many Valence Electrons In C2h6
A carbon atom has 4 valance electrons and it needs 4 more electrons to complete its octet. On the periodic table Carbon is in group 4 or 14 so it has 4 valence electrons but we have 2 of them.

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen
Besides what is the special feature of the structure of ethane.
How many valence electrons in c2h6. After determining how many valence electrons there are in C2H6 place them around the central atom to complete the octets. We have 6 multiply that by 6 for a total of 14 valence electrons to work with. Since there are 6 carbon atoms multiply 4 by 6 to get 24.
14 Votes In the Lewis structure of C2O42- there are a total of 34 valence electrons. C 6 H 6 has a total of 18 valence electrons. This means that the Lewis dot structure for C2H 6 must account for 14 valence electrons either through bonding between atoms or through lone pairs.
Include all hydrogen atoms. Since C has 4 valence electrons and each H atoms contributes 1 valence electron the total number of electrons will be 24 61 14 e- This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons either through. Drawing the Lewis Structure for C 2 H 2 Ethyne or Acetylene For C 2 H 2 you have a total of 10 valence electrons to work with.
Hydrogen is the first element in the periodic table therefore it has only one valence electron. The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. Each C atom forms three covalent bonds with three H atoms with one aditional covalent bond being formed between the two C atoms.
Since C has 4 valence electrons and each H atoms contributes 1 valence electron the total number of electrons will be 24 61 14 e - This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons either through. In ethane we have two carbon atoms and 6 hydrogen atoms and hence the total number of valence electron are 2 X 4 1 X 6 14. How can I write the Lewis dot structure for C2H6How many valence electrons are in an atom of phosphorusHow do you find all factors of 54John got 12 marbles that are red and 34 that are blue what is the probability get 3 blue marblesWhen sinx1 what does x equalWhat are the most reactive metals according to the metal activity seriesHow many resonance structures are there for NO2What.
Lets do the Lewis structure for C2H6 ethane. The tendency to form species that have eight electrons in the valence shell is called the octet rule. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.
The C2H6 Lewis structure has a total of 14 valence electrons. In the case of carbon we have four valence electrons each. So lets multiply that times 2.
Hydrogen H atoms always go on the outside of a Lewis structure. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. Hydrogen H atoms always go on the outside of a Lewis structure.
How Many Valence Electrons Does NCl3 Nitrogen Trichloride Have. And then Hydrogen group 1 one valence electron. If playback doesnt begin shortly try restarting your device.
The C2H6 Lewis structure has a total of 14 valence electrons. Ethane exists in nature as a flammable colorless and odorless gas. Since the noble gas before carbon is Helium you subtract 2 electrons from 6 electrons which gives you 4 valence electrons.
Drawing the Lewis structure. The total number of valence electrons in one molecule of C2H4. 455 1145 Views.
So the two C atoms are placed in the center of the molecule. Click to see full answer.

Is Chf3 Polar Or Nonpolar Fluoroform In 2021 How To Find Out Molecules Hydrogen Atom

Molar Mass Of Nh4cl Ammonium Chloride In 2021 Molar Mass Molars Molecules

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation

Is C2h6 Polar Or Non Polar Ethane In 2021 Math Equations Chemical Formula Molecules

N3 Lewis Structure Azide Ion In 2021 Math Equations Lewis Molecules

Is Of2 Polar Or Nonpolar Oxygen Difluoride In 2021 Oxygen Chemical Formula Molecules

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules

Hydrocarbons Math Equations Chemistry Math

Igcse Identifying Ionic Covalent Bonds Covalent Bonding Ionic And Covalent Bonds Covalent Bonding Worksheet

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Cs2 Lewis Structure Carbon Disulfide In 2021 Lewis Math Equations Molecules
C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape
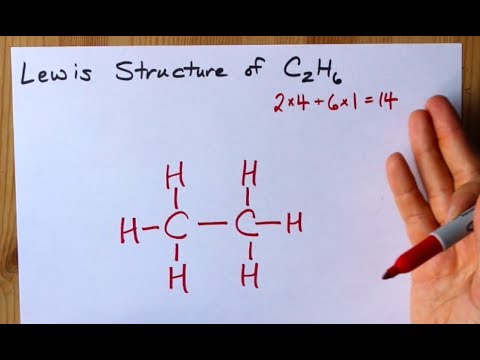
Lewis Structure Of C2h6 Ethane Youtube

Welcome To Learnapchemistry Com Bond Length Molecular Geometry Covalent Bonding

What Are Hydrocarbons Gulf Coast Environmental Systems Chemistry Organic Chemistry Chemistry Classroom

Figura 1 Notacao De Lewis Para Os Atomos Neutros De Hidrogenio E Carbono E Para As Moleculas De Agua Etileno Eteno Chemical Bond Chemistry Notes Chemistry
