How To Determine Lewis Structure Shape
The atomic number of Hydrogen H is 1 so its electronic configuration is 1s1. Lone pair on the central atom.
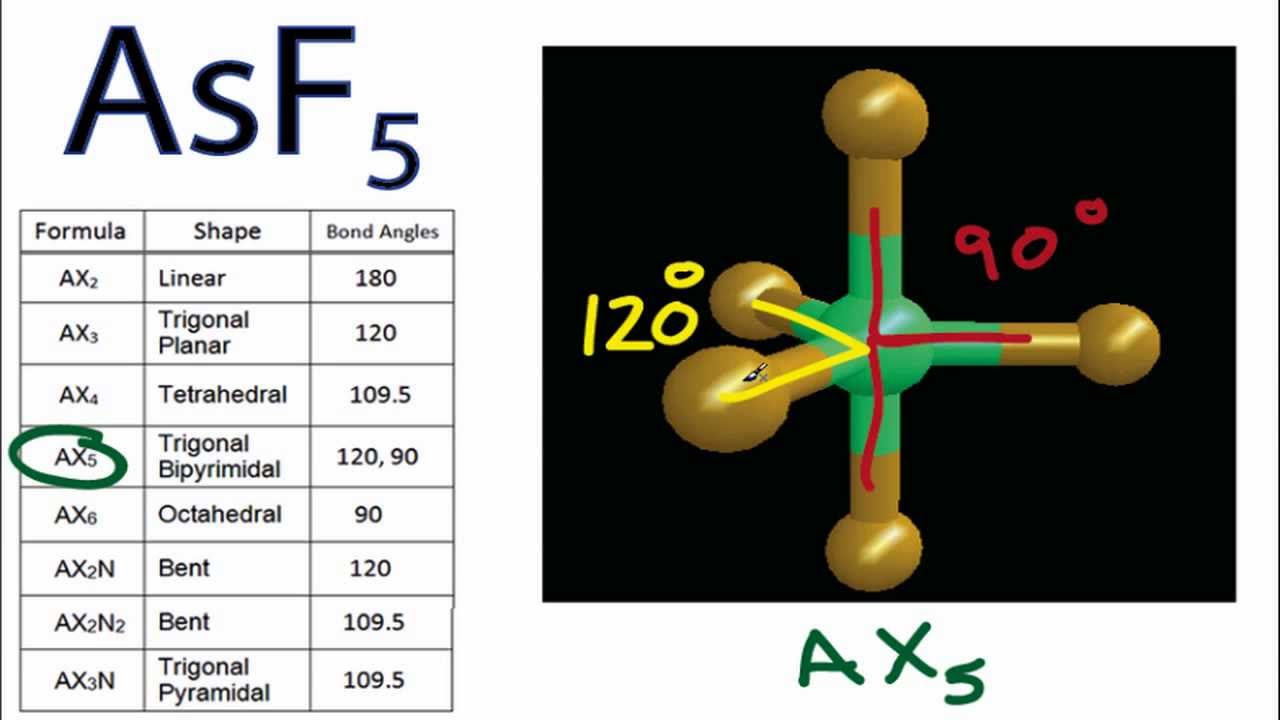
Asf5 Molecular Geometry And Bond Angles Arsenic Pentafluoride Molecular Geometry Molecular Geometry
Ketzbook explains molecular geometry VSEPR theory and the 5 basic shapes of molecules with examples for each one.

How to determine lewis structure shape. Determine the Lewis structure VSEPR and the name of the shape for the following. Electron pairs gives base shape Octahedral VSEPR base shape for 6 e-pairs Refcode. Remember electron groups include.
That gives you the steric number SN the number of bond pairs and lone pairs around the central atom. For molecules of the formula AX n place the atom with the. Watch to the end to learn how you can ge.
If a molecule has more than one element add the valence electron of all elements present in the compound. For example H 2 O has 2x1 6 8 valence electrons CCl 4 has 4. A XeF4 b SCl2 By signing up youll get thousands.
There are three basic steps to determining the molecular shape of a molecule. Write the Lewis structure of the molecule or polyatomic ion. Determine the total number of valence electrons of the element or compound.
The central atom is the least most electronegative atom in the compound. Lewis Structures and the Shapes of Molecules. See full answer below.
CCl 2 F 2 d. Count the total number of valence electrons in the molecule or polyatomic ion. N 2 O i.
The structure on the right is the Lewis electron structure or Lewis structure for H 2 O. Determine which atom will be the central atom of the Lewis Dot Structure. Add or subtract electrons for charge see Top Tip 5.
Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons. With two bonding pairs and two lone pairs the oxygen atom has now completed its octet. First of all we need to find how many lone pairs ClF3 central atom contains.
H 2 O m. As we see in the ClF3 lewis structure Chlorine which is the central atom contains 2 lone pairs. CF 2 H 2 e.
A Lewis dot structure is a two-dimensional sketch of a molecule that uses dots to represent valence electrons. Name the electron-group geometry. Identify the central atom 2.
The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular geometries. Become a member and. A single double or triple bond counts as one region of electron density.
Steps Used to Find the Shape of the Molecule. Write the Lewis dot structure of the molecule. Add one electron for each bonding atom 4.
You can determine the molecular geometry from the Lewis structure of a molecule by determining the number of lone pairs on the central atom as well. State whether it is linear. As the s shell needs two electrons there is a vacancy of one electron so the number of valence electrons in one Hydrogen H atom is 1.
A Lewis structure representation is the simplest way to indicate the chemical formula of a compound by showing its valence and bonding electrons along with the formal charge. These electrons are the ones that are present in the outermost shell of the atom and participate in forming bonds. Or we can find lone pair in ClF3 by using the direct formula.
LP VE. Divide the total of these by 2 to find the total number of electron pairs. Dimensional molecular models to determine the shape of the molecules.
Draw the Lewis Structure. Lewis Structures Shapes and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules. Count its valence electrons 3.
CH 2 O f. You will also examine how bonding and shape can explain whether a molecule is polar or non-polar. Use the SN and VSEPR theory to determine the electron pair geometry of the molecule.
Count the number of regions of electron density lone pairs and bonds around the central atom. For determining the Lewis Structure for any molecule we first need to know the total number of valence electrons. Place the atoms relative to each other.
To begin with the Lewis structure of CH3Cl first we need to determine the electronic configuration of each participating atom. Find the Number of lone pairs present on the central atom of the ClF3 lewis structure. Moreover by sharing a bonding pair with oxygen each hydrogen atom now has a full valence shell of two electrons.

Bent Molecular Geometry Molecular Shapes Molecular Geometry Chemistry Projects

So32 Molecular Geometry Shape And Bond Angles Molecular Geometry Chemistry Help Molecular

Ch2cl2 Lewis Structure Dichloromethane In 2021 Molecules Math Equations Hydrogen Atom

Tetrahedral Molecular Geometry Molecular Geometry Chemistry Projects Nomenclature Chemistry

Vsepr Theory What Is It Importance Limitation Notation Videos Q A Molecular Geometry Vsepr Theory Molecular Shapes

Is Hcn Polar Or Nonpolar Hydrogen Cyanide In 2021 Chemical Formula Molecules Polar

Draw A Lewis Structure Of Formaldehyde Lewis Chemistry Draw

Ch4 Lewis Structure Methane In 2021 Lewis Methane Chemical Formula

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 In 2021 Lewis Octet Rule Structures

Is No3 Polar Or Nonpolar Nitrate In 2021 How To Find Out Molecules Polar

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry

Vsepr Theory Vsepr Theory Organic Chemistry Chemistry

Trigonal Pyramid Molecular Geometry Molecular Geometry Molecular Shapes Chemistry Projects

Lewis Dot Structure Worksheet Vsepr Origami Worksheet Post Lab Answers Kids Worksheets Printables Geometry Worksheets Math Addition Worksheets

Image Result For Molecular Geometry Chart Molecular Geometry Teaching Chemistry Chemistry Lessons

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Study

