Lewis Dot Structure For C2h2cl2
This is the C2H2Cl2 Lewis structure. Download C2H2Cl2 Lewis Structure Molecular Geometry Images.

There Are 3 Lewis Structures For C2h2cl2 D Clutch Prep
For the molecule we expect the carbons to be the central atoms in this molecule since carbon tends to be the central atom in its compounds.

Lewis dot structure for c2h2cl2. Carbon has 4 valence electrons two Carbons. Hydrogen has 1 valence electron but we have two Hydrogens. The Lewis structure also represents the electron dot structure or an electron dot diagram of.
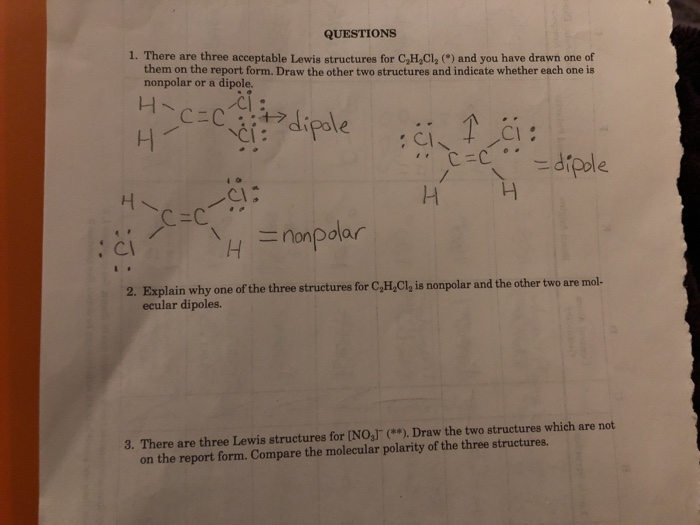
The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms. You can smell very small amounts of 1 2-dichloroethene in air about 17 parts of 1 2-dichloroethene per million parts of air 17 ppmThere are two forms of 1 2-dichloroethene. Draw the other two structures and indicate whether each one is nonpolar or a dipole.
Harmful if swallowed Warning Acute toxicity oralH315 100. A step-by-step explanation of how to draw the C2H2Cl2 Lewis Dot Structure 12-DichloroetheneFor the C2H2Cl2 structure use the periodic table to find the t. A Draw Lewis structures of the three isomers all of which have a carboncarbon double.
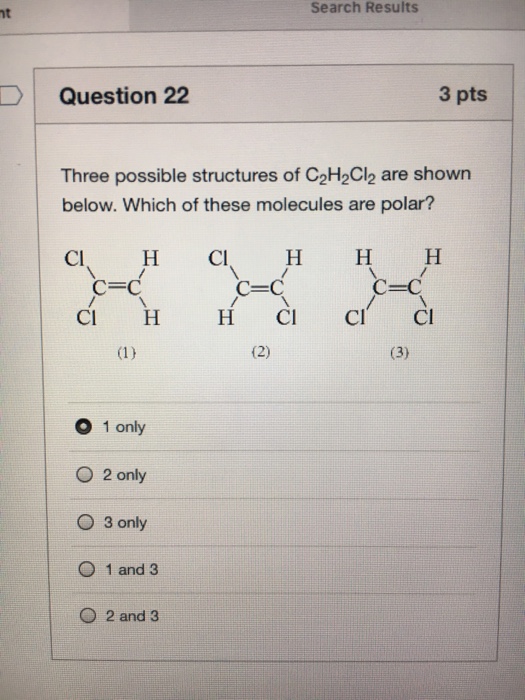
There are three possible compounds isomers with the formula C2H2Cl2. Jiskha Homework Help - Search. There are 3 Lewis structures for C 2 H 2 Cl 2 draw all three and indicate whether each is non-polar or polar.
Draw the Lewis Structure of the molecule C2H2Cl2. In lewis structure the lines represent the bonds and dots represent the valence electrons. There are three acceptable lewis structures for c2h2cl2 and you have drawn one of them on the report.
Calculate the total number of valence electrons present. Actually no there is only one acceptable Lewis structure for CHmath_2mathClmath_2math Moving the chlorines around does not produce a new compound with a. Determine the central atom in this molecule.
Draw the Lewis structure for the molecule. Molecular geometry of trans-difluroethylene. For C2H2 Lewis structure we will first place both the Carbon atoms in the centre as it is less electronegative than the Hydrogen atoms.
If you look at the Hydrogen atoms it only needs one valence electron to attain a stable structure. What is the lewis structure C2H2Cl2. There are 2 Hydrogen atom s 2 Carbon atom s and 2 Chlorine atom s.
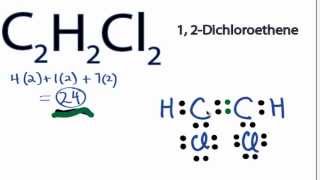
Vinylidene chloride H2CCCl2 or C2H2Cl2 CID 6366 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Then learn how to predict the shape of a molecule by applying the VSEPR theory to the Lewis dot structure. Plus Chlorine which is 7 and we have two Chlorines for a total of 24 valence electrons.
For C 2 H 2 you have a total of 10 valence electrons to work with. Draw three Lewis structures for compounds with the formula C 2 H 2 F 2. In lewis structure the lines represent the bonds and dots represent the valence electrons.
Draw and explain the Lewis structure for C2H2Cl2. Lewis structure is the lewis dot structure and a electron dot diagram the same thing. Explain why one of the three structures for CHCl is nonpolar and the other two are mol ecular dipoles.
The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2. Carbons the least electronegative and well put that in the center and we know Hydrogens always go on the outside. We have 24 valence electrons for the C2H2Cl2 Lewis structure.
In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. Here both the Carbon atoms take the central position and the Hydrogen atoms are arranged around it. The chemical formula of cis-12-Dichloroethene shown above is based on the molecular formula indicating the numbers of each type of atom in a molecule without structural.
There are three acceptable Lewis structures for CaH Cl2 and you have drawn one of them on the report form. Methylene chloride also known as Dichloromethane DCM is an organic chemical compound. The valency of carbon is four so it likes to have exactly four bonds so if the each carbon atom is bonded to two non - carbon atoms the.
Were being asked to draw the 3 Lewis structures for C 2 H 2 Cl 2 and indicate whether each is non-polar or polar. I know how to draw the lewis structure. The cis-12-Dichloroethene molecule contains a total of 6 atom s.
Answers What is the Lewis Dot Structure for C6H6 and both for C2H2Cl2. To do so we first need to do the following steps. Indicate which of the compound s are polar.
A chemical formula of cis-12-Dichloroethene can therefore be written as.
Laboratory 11 Molecular Compounds And Lewis Structures Flip Ebook Pages 1 10 Anyflip Anyflip
Search Results Nt Question 22 3 Pts Three Possible Chegg Com

How To Determine The Lewis Dot Structure For C2h2cl2 Quora

C2h2cl2 Lewis Structure How To Draw The Lewis Structure For C2h2cl2 Youtube
Olid Or A Liquid Laboratory Paper Chr 1 Is Xefs Chegg Com
C2h2cl2 Lewis Structure How To Draw The Lewis Structure For C2h2cl2 Youtube
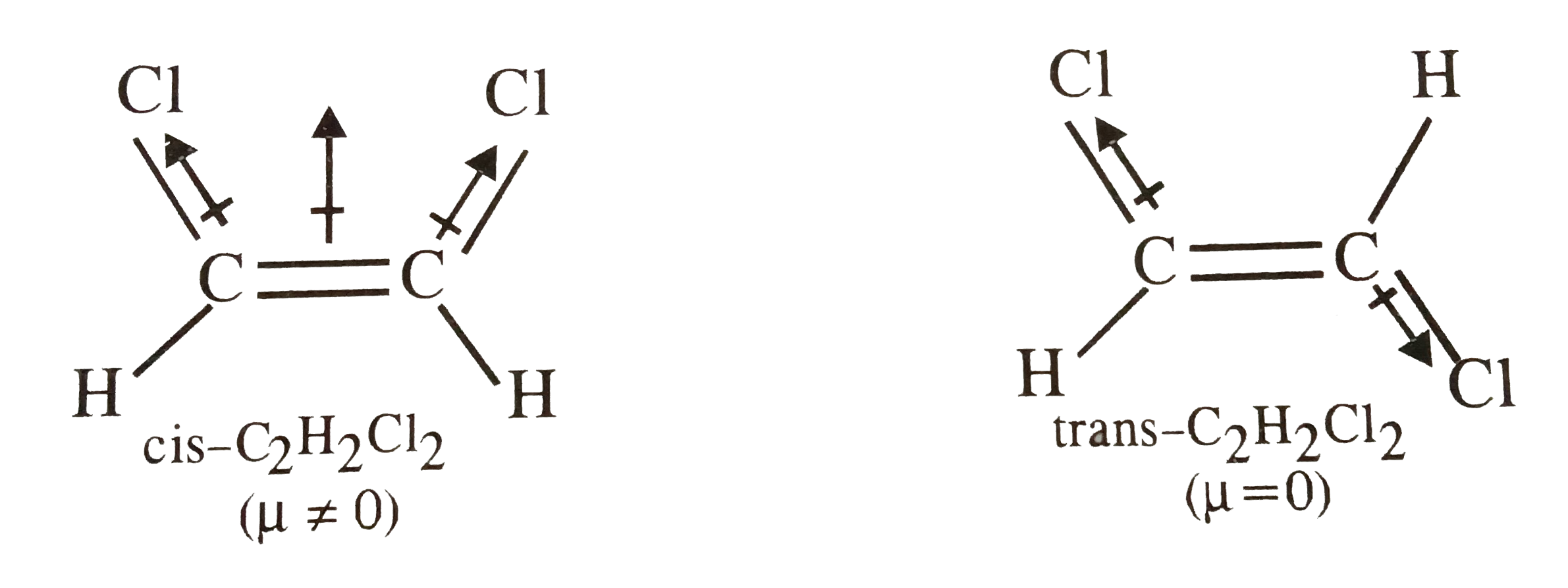
Sketch The Bond Moments And Resultant Dipole Moments In Cis And
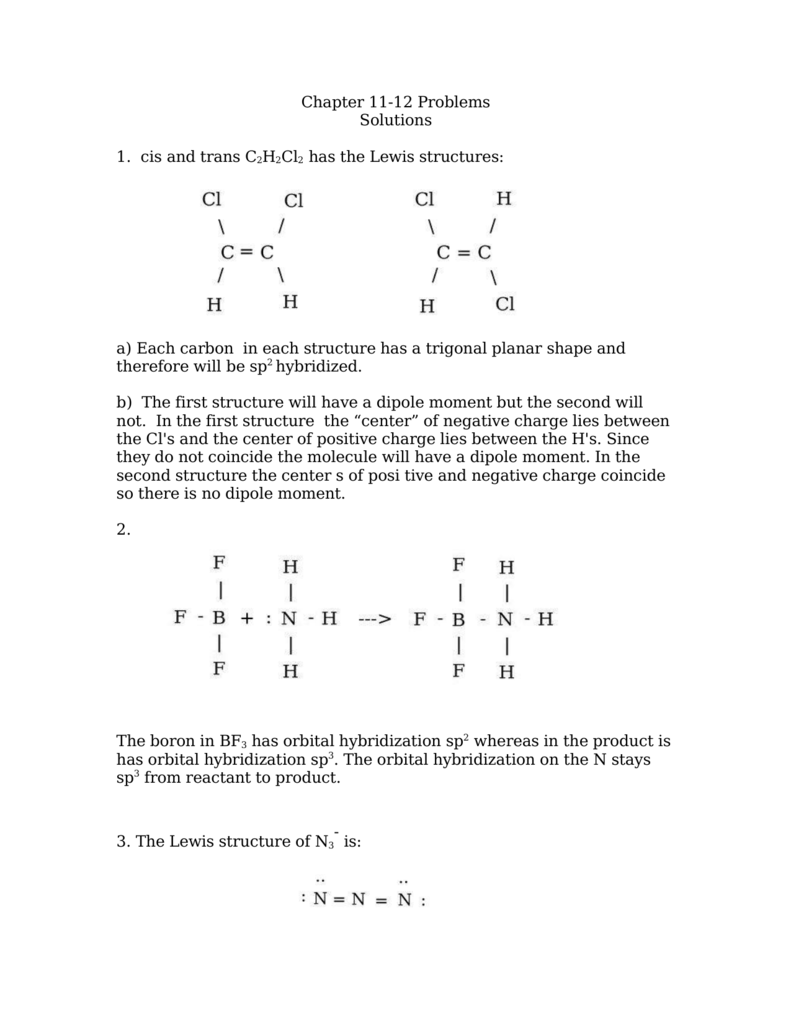
Chapter 11 12 Problems Solutions 1 Cis And Trans C2h2cl2 Has The

State True Or False Two Isomers Of C2h2cl2 Are Polar

Dichloroethylene C2h2cl2 Has Three Forms Clutch Prep

C2h2br2 Lewis Structure How To Draw The Lewis Structure For C2h2br2 Youtube

There Are 3 Lewis Structures For C2h2cl2 D Clutch Prep

C2h2cl2 Lewis Structure How To Draw The Lewis Structure For C2h2cl2 Youtube

C2h2cl2 Lewis Structure Homeworkavid
There Are 3 Acceptable Lewis Structures For Ch2cl2 What Are The 3 Lewis Structures Quora
Explain Why One Of The Three Structures For C2h2cl2 Chegg Com

Why One Of The Three Structures For C2h2cl2 Is Nonpolar Brainly Com