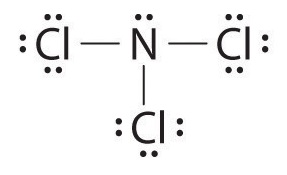
Ncl3 Lewis Structure Shape
Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. This is mainly formed as a by-product when chlorine is treated with the ammonia derivative compounds.
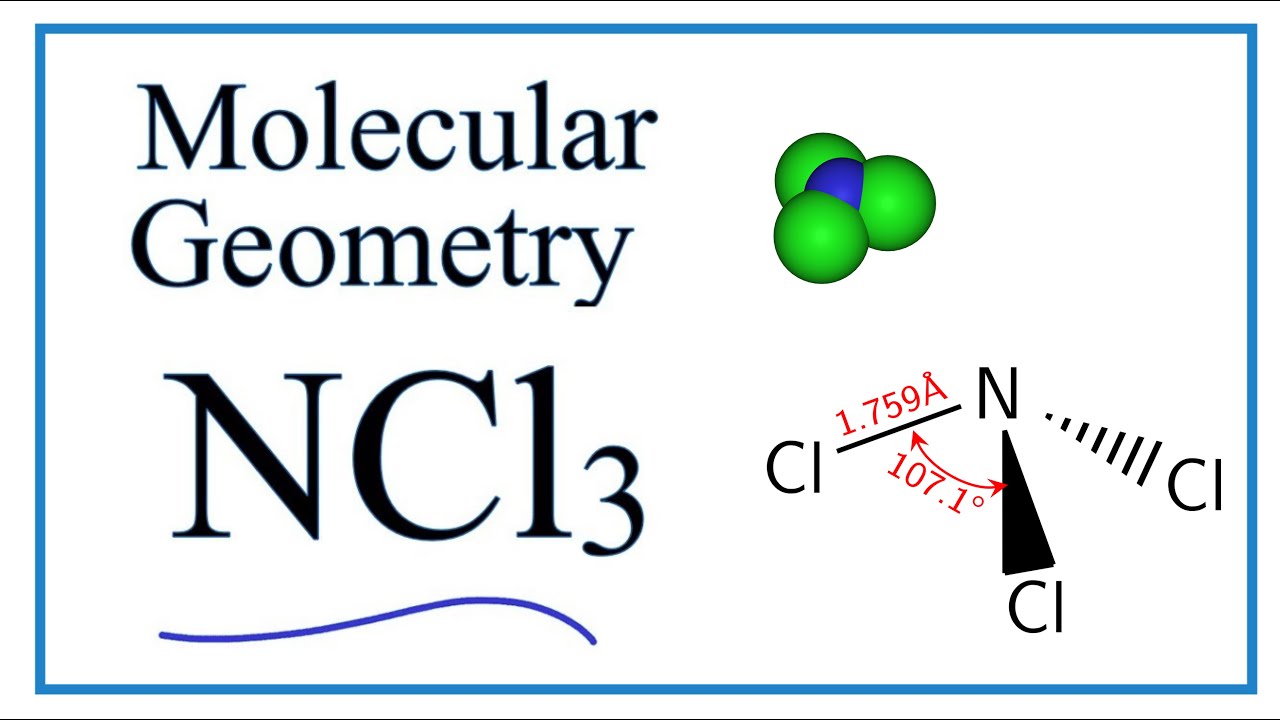
Ncl3 Molecular Geometry Shape And Bond Angles Youtube
Draw The Lewis Structure For TeCl4.

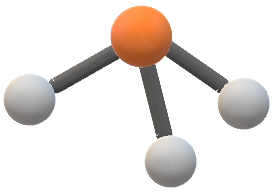
Ncl3 lewis structure shape. We need to draw a skeletal structure with single bonds only. An NCl3 molecule would be a trigonal pyramidal because it has one center N atom with 3 Cl surrounding it but also a lone pair of electrons on the top which bends the molecule downward forming a trigonal pyramidal. Assuming that biological substances are 98 water estimate the masses of b a cell with a diameter of 10 μm c a human kidney and d a.
NCl3 Nitrogen trichloride Lewis Structure. Its electron shape would be tetrahedral that is when you count the lone pairs of electrons as bonds themselves. Draw the Lewis structure for NCl3.
Determine the electron geometry eg and molecular geometry mg of NCl3. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. Now boron is less electronegative which makes it the central atom.
This pair exerts repulsive forces on the bonding pairs of electrons. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is distorted because of the lone pairs of electrons.
By signing up youll get. In NCIS the central atom is nitrogen and the terminal atoms are chlorine atom. Draw The Lewis Structure For NCl3.
Chemical formula Lewis structure Geometric sketch including bond angles CH4 OCl2 NCl3 CO2 CH2O. The proposed Impinger Method IM was used to measure the environmental levels of nitrogen trichloride NCl3 in 17 indoor swimming pools located in Northern Italy. BCl3 Lewis Structure.
Include lone pairs NCl3. Lewis structure of Nitrogen trichloride contains 1 lone pair and 3. Were asked to draw the Lewis dot structure for NCl 3.
It is a volatile liquid that reacts with water and releases HCl gas. In the Lewis structure for NCl 3 there are a total of 26 valence electrons. Nitrogen trichloride is a yellow oily liquid with its pungent odor.
Lewis dot structures are useful to predict the geometry of a molecule. The hybridization of NCl3 is Sp³. Nitrogen trichloride or NCl3 is arranged in a tetrahedral structure with one single lone pair located on the Nitrogen atom.
A eg tetrahedral mg trigonal pyramidal B eg linear mg trigonal planar. If you can do those Lewis structures NCl 3 will be easy. Drawing the Lewis Structure for NCl 3.
For NCl3 draw the Lewis structure predict the shape and determine if the molecule is polar or nonpolar. This new analytical protocol is based on a colorimetric reaction commonly employed to detect the total and free chlorine levels in water. 40-10 30e-15 lone pairs.
First of all we need to calculate the total valence electrons of this molecule B 3 C l 7 3Cl 7321 So total 213 24. NCl 3 is similar to NH 3 and NF 3. The molecular geometry of NCl3 is trigonal pyramidal and its electron geometry is tetrahedral.
Three pairs will be used in the chemical bonds between the N and Cl and one pair of. Complete the following table by first drawing a Lewis structure and geometric sketch for each formula. PCl3 Molecular Electron Geometry Lewis Structure Bond Angles and Hybridization.
NCl3 molecule has one lone pair that leads to repulsion between electrons and the shape of the molecule is trional pyramidal. In the NH 3 Lewis structure Nitrogen N is the least electronegative so it goes in the center. ABCD or E below.
Nitrogen trichloride is slightly polar in nature. 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3. The first one has been completed as an example.
The total valence electron available for the NCl3 lewis dot structure is 26. What Is The ARRANGEMENT For This Structure. Unlike protons and neutrons valence electrons take part in the excitement of a chemical reaction.
Predict the electron geometry and molecular geometry and state whether the molecule is polar or. Nitrogen trichloride NCl3 lewis structure contains three N-Cl bonds. Let us apply the lewis dot rules and try to draw the structure of boron trichloride.

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
What Is The Molecular Geometry Of Ncl3 Quora

The Total Number Of Lone Pairs In Ncl3 Is Study Com
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

Determine The Electron Geometry Eg And M Clutch Prep

Nitrogen Trichloride Ncl3 Lewis Dot Structure Youtube

Draw The Lewis Structure For Ncl3 And Provide The Following Information A Number Of Electron Groups B Electron Pair Geometry C Bond Angle D Number Of Bonded Electrons E Molecular Geometry F
Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Determine The Electron Geometry Eg And M Clutch Prep

Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube
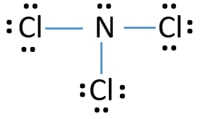
Ncl3 Nitrogen Trichloride Lewis Structure

Choose A Lewis Structure For Ncl3 Clutch Prep

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Is Ncl3 Polar Or Nonpolar Techiescientist