What Is The Electron Geometry Of Xef2
Determine the electron geometry eg and molecular geometry mg of XeF2. For the above molecule VSEPR notation will be AX 2 E 3.
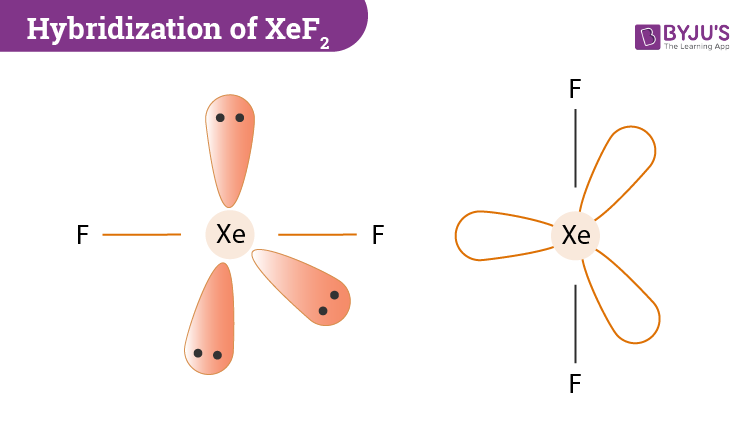
Hybridization Of Xef2 Hybridization Of Xe In Xenon Difluoride
Determine the electron geometry eg and molecular geometry mg of XeF2 A egtrigonal bipyramidal mgbent B eglinear mglinear C egtetrahedral mglinear D egtrigonal bipyramidal mglinear E egtetrahedral mgbent.
What is the electron geometry of xef2. This corresponds to AX5 or trigonal bipyramidal. XeF2 Molecular Geometry And Bond Angles. B What is the electron-pair geometry for Xe in XeF4.
The lone pairs are on the equatorial position to the bonded pairs. The electron geometry of xenon difluoride is trigonal bipyramidal molecular geometry is linear. What is the molecular geometry of SF4.
What is the molecular geometry of SCl4. It acquires such shape as the lone pairs present around the central atom tend to take up equatorial positions. The bond angle is said to be 180.
A eg trigonal bipyramidal mg-bent B eg linear mg linear C eg-tetrahedral mg linear D eg-trigonal bipyramidal mg linear E eg-tetrahedral mg-bert 3. What is the electron geometry of ClF3. Physical Properties of Xenon Difluoride XeF 2.
The molecule is categorized as linear as the bonded pairs float around the central Xe atom except the lone pairs. See the answer See the answer See the answer done loading. According to the VSEPR theory The molecular geometry of the molecule is linear.
What electron geometry is associated with sp3d2 hybridization. For todays video we will learn the. AX 2 E 3 has linear shape.
What is the the shape molecular. The structure of xenon difluoride is illustrated below. What is the electron geometry of PCl5.
B What is the electron-pair geometry for N in NHCI. The bond angle of F-Xe-F is 180 degrees. XeF2 structure features two covalent bonds between one xenon atom and two fluorine atoms.
IXeF2 is a linear moleculeIIXe is the only group VIIIA element to form molecules with other elementsIIIXeF4 is a planar molecule. Electron groups include lone pairs and atoms around the central atom. The electron geometry is represented by.
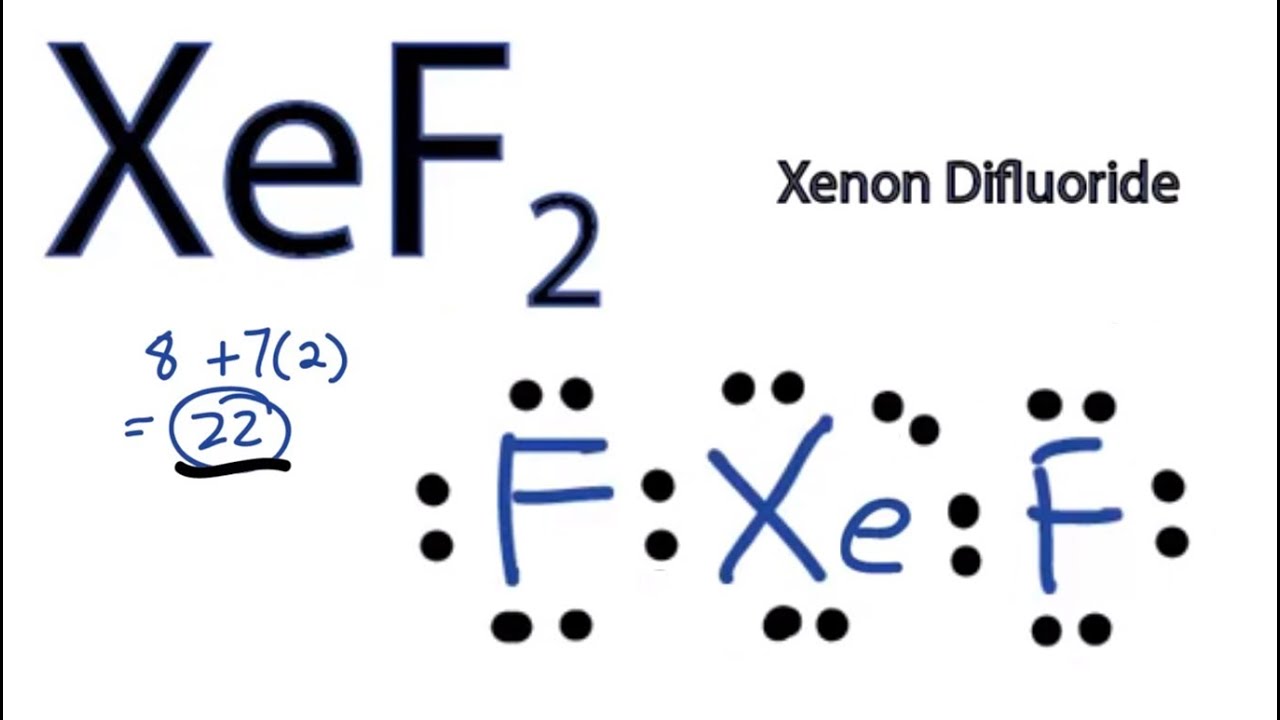
To summarize the article it can be concluded that XeF2 has 22 valence electrons out of which there are three lone pairs of electrons. We are back with a video that will help you determine the molecular geometry of any given molecule in no time. The electron geometry is octahedral while the molecular geometry is square planar Xenon has 6 bonding electron pairs therefore the electron geometry of octahedral but two of the pairs of electrons on the central atom are unbonded or lone pairs therefore the molecular geometry is square planar.
Chemistry questions and answers. XeF2 Lewis Structure Lewis Structure also known as electron dot structure is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule. The electron bonded pairs take the apex position in this trigonal bi-pyramid giving rise to linear molecule.
So the shape of XeF 2 is linear. The bond angle between the two pairs bonded with the central atom is 180 degrees which makes the molecular geometry of XeF2 linear. What is the electron geometry for XeF2.
C What is the the shape molecular geometry of NHCI. Asked Mar 25 2019 in Chemistry by joecar general-chemistry. Read More About Hybridization of Other Chemical Compounds.
Xenon is an inert gas element. XeF2 molecular geometry is linear. This problem has been solved.
Its hybridization is sp3d. Submit What is the the shape molecular geometry of XeF2. We use dots to represent outer shell electrons and lines to represent the bond type.
What is the electron geometry of XeF2. Use VSEPR table to find the shape. The xenon atom also holds 3 lone pairs of electrons.
For SCl4 its electron pair geometry is trigonal bipyramidal AX5. For SCl4 there are 1 lone pair and 4 atoms or a total of 5 electron groups around S. Download a copy of VSEPR shapes table here.
See full answer below. There are two pairs of bonded electrons and three lone pairs of electrons. What is the electron geometry of ICl5.
The molecular geometry of XEF2 is the non bonded pair of electrons amounting to three give rise to equatorial position. Determine the electron geometry eg and molecular geometry mg of XeF4.

Xef2 Lewis Structure Molecular Geometry Youtube
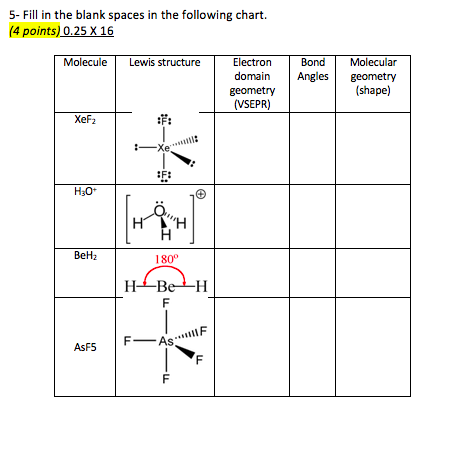
Bond Angles Molecular Geometry Shape Fas F 5 Fill Chegg Com

6 14 Determine The Electron Pair And Molecular Geometries For Xef2 University Of Kentucky Youtube
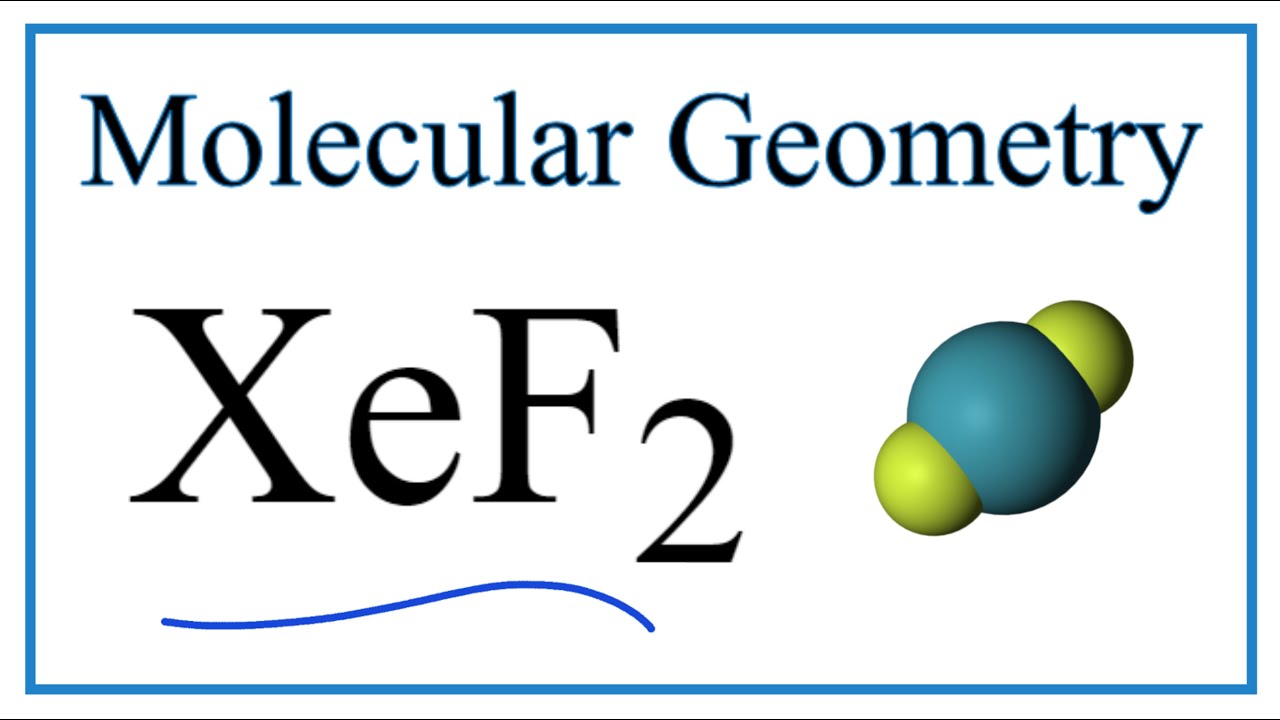
Xef2 Molecular Geometry Bond Angles Electron Geometry Youtube

What Is The Molecular Shape Of Xef2 Quora

What Is The Shape Of Xef2 Quora

What Is The Molecular Shape Of Xef2 Quora
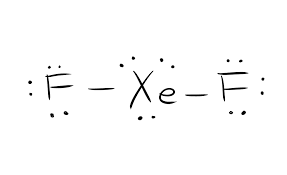
Xef2 Lewis Structure Polarity Hybridization And Shape

Xef2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Xef2 Lewis Structure Polarity Hybridization And Shape
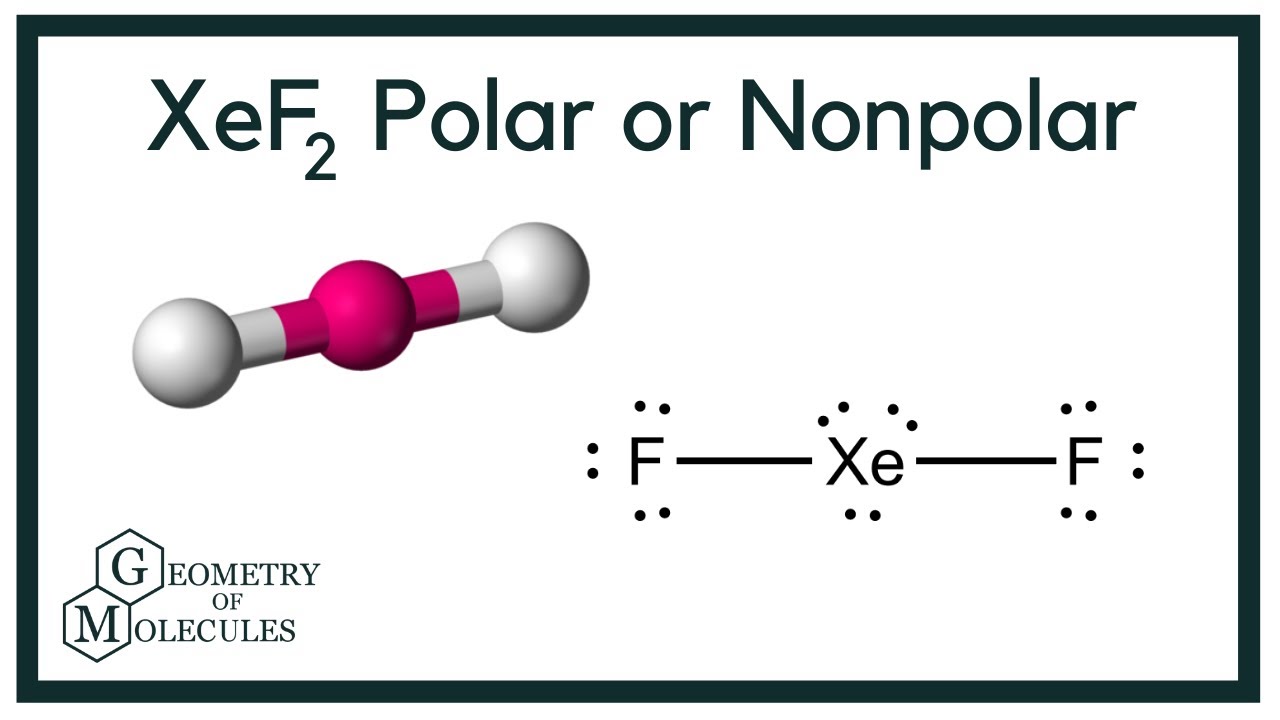
Xef2 Lewis Structure Polarity Hybridization And Shape
What Is The Shape Of The Xef2 Molecule And The Total Number Of The Lone Pair Present On Xe In A Xef2 Molecule Quora

Xef2 Lewis Structure Molecular Geometry Youtube
What Is The Molecular Shape Of Xef2 Quora
Does The Vsepr Theory Predict That Xef2 Is Linear Socratic

Hybridization Of Xef2 Hybridization Of Xef6

Xef2 Molecular Geometry Bond Angles Electron Geometry Youtube

What Is The Molecular Shape Of Xef2 Quora
