What Is The Lewis Structure For N2h4
We first need to determine the total number of valence electrons present in the molecule. Most blogs that I had looked at did not have the Lewis Structure.

Hydrazine N2h4 And Carbon Disulfide Cs2 Clutch Prep
C Draw The Orbital Diagram For The Valence Electrons Of Nitrogen In A N2H4 Molecule Below.

What is the lewis structure for n2h4. The first picture the picture of the Lewis Structure of Hydrazine was accurate and very unique to the blog. Hydrogen H only needs two valence electrons to have a full outer shell. N 5A 2 5 e 10 e H 1A 4 1 e 4 e Total.
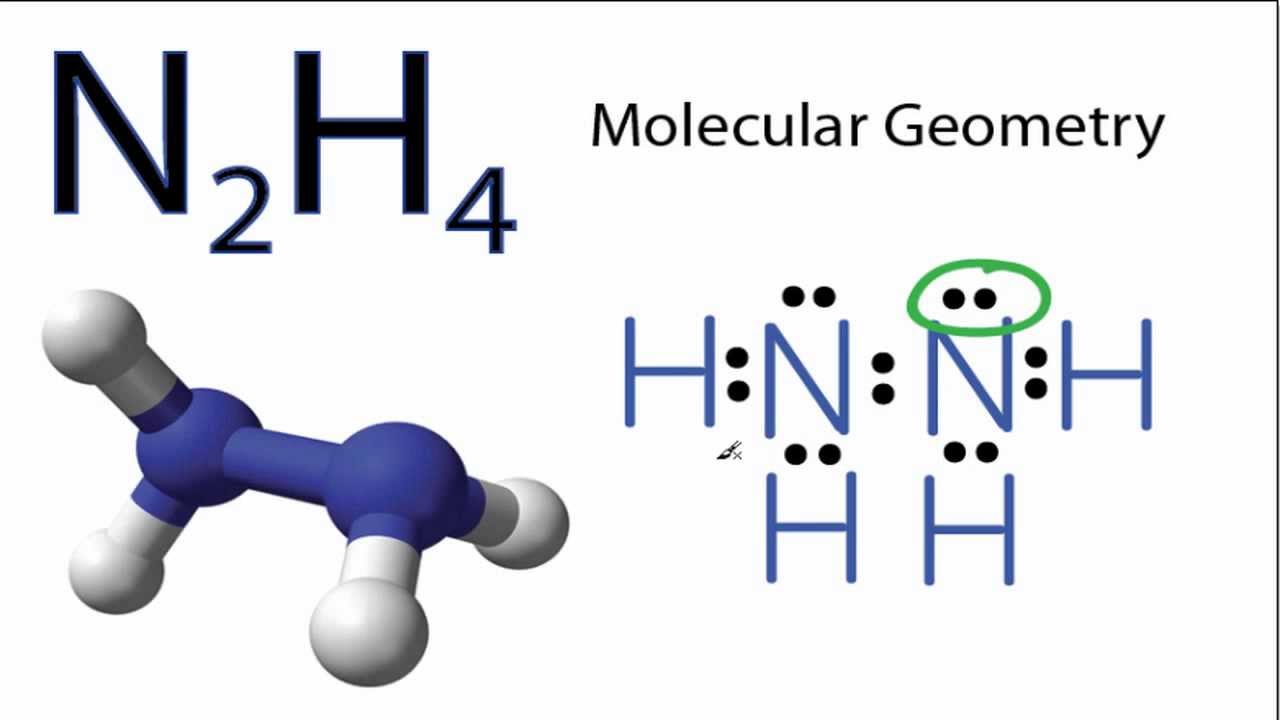
Lewis structure of N2H4 is made up of two nitrogen and four hydrogens having two lone pairs on the nitrogen atomsone lone pair on each nitrogen and contain a total of 10 shared electrons. Draw the lewis dot structure of n2h2. There are three types of bonds present in the lewis.
In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. The somewhat darker turquoise gave the blog a sleek and simple look which I really liked. The two atomic orbitals aos involved in the formation of a sigma bond between two hydrogen atoms in the molecule h2 are draw.
See full answer below. It can adapt two different geometrical forms cis and trans each with distinct symmetry. Note The Relative Energies Of The Orbitals.
Put atoms with lowest electro negativity in the center. Drawing resonance structures following the lewis structure. N 2 H 4 is straightforward with no double or triple bonds.
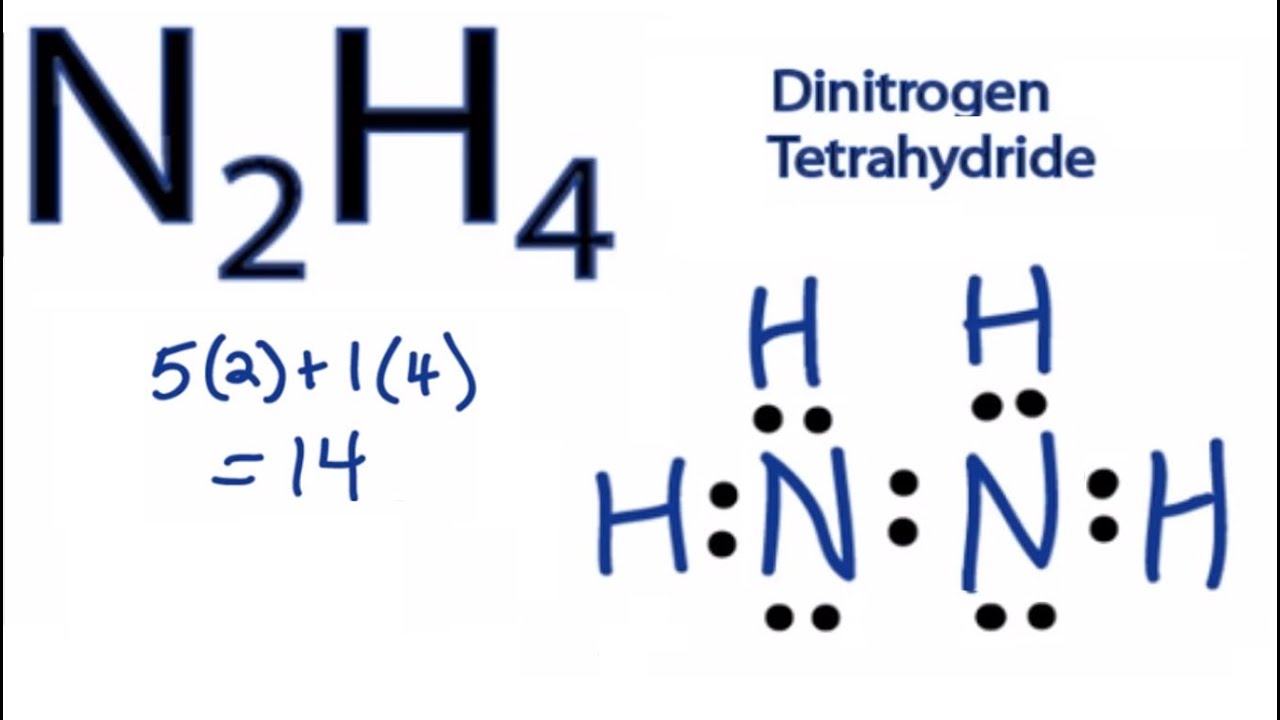
N2H4 is straightforward with no double or triple bonds. Lewis structure of N 2 H 4 In Lewis structure a shared electron pair between two bonding. In the Lewis structure for N2H4 there are a total of 14 valence electrons.
Back in the center twelve and fourteen. A step-by-step explanation of how to draw the N2H4 Lewis Dot Structure HydrizineFor the N2H4 structure use the periodic table to find the total number of. There are 12 valence electrons for this molecule.
N 2 O nitrous oxide is an oxide of nitrogen and is called as laughing gas. Identify The Correct Lewis Structure For N2H4. Were being asked to draw a Lewis structure for N 2 H 4.
Hydrogen H only needs two valence electrons to have a full outer shell. A Draw The Lewis Structure For Hydrazine N2H4 B List The Electronic And Molecular Geometries For NH3. N 2 O Lewis Structure Resonance Structures Oxidation Number.
Arrange the remaining atoms around it. It has four bonding pairs of electrons and two lone pairs of electrons. How to draw the lewis structure for N 2 O.
Lewis structure for N 2 H 4 is as shown in the figure. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. What is the Lewis structure for N2H4.
N2h2 n2h4 c2h2 c2h4 h3coch3 by signing up youll get thousands of. A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc. Lets do the N2H4 Lewis structure.
Hydrogen H only needs two valence electrons to have a full outer shell. Hydrogen is usually surrounded by 4 electrons in a valid lewis structure. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the ou Weve used six eight ten.
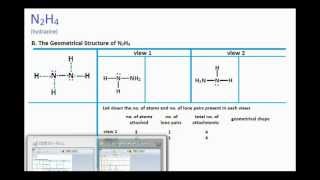
The molecule has a trigonal pyramidal shape with a bond angle less than 1095. Chemistry questions and answers. Each Nitrogen atom forms a single bond with one Hydrogen atom and a double bond with the neighboring Nitrogen atom.
Hydrogen only needs two for a full outer shell so all of our Hydrogens are OK. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
Use information from step 4 and 5 to draw the lewis structure. N2H4 is straightforward with no double or triple bonds. Hydrogen H only needs two valence electrons to have a full outer shell.
14 valence e. What Is The Hybridization Of N In Hydrazine. N2H4 is straightforward with no double or triple bonds.
Label The Orbitals Appropriately. In this tutorial we are going to learn followings of nitrous oxide gas. Finally put the bond pairs and lone pairs of electrons on the atoms.
Determine the molecular geometry of N2H4 skeletal structure The molecular geometry is about each interior nitrogen atom of n2h4 is Trigonal pyramidal. Next was the 3-D structure. In the Lewis structure for N2H4 there are a.
In the Lewis structure for N2H4 there are a total of 14 valence electrons. Identify The Correct Lewis Structure For N2H4. The lewis structure for n2h2 hhnh shows1.
In the Lewis structure for N 2 H 4.

Draw The Lewis Structure For N2h4 Clutch Prep

Hydrazine N2h4 Hydrazine Polar Molecule
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube

Nh2nh2 Lewis Structure How To Draw The Lewis Structure For Hydrazine Youtube
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

Hydrazine N2h4 Lewis Structure

Part D Draw The Lewis Structures Of N2h4 Clutch Prep

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
N2h4 Lewis Structure And Molecular Geometry Youtube
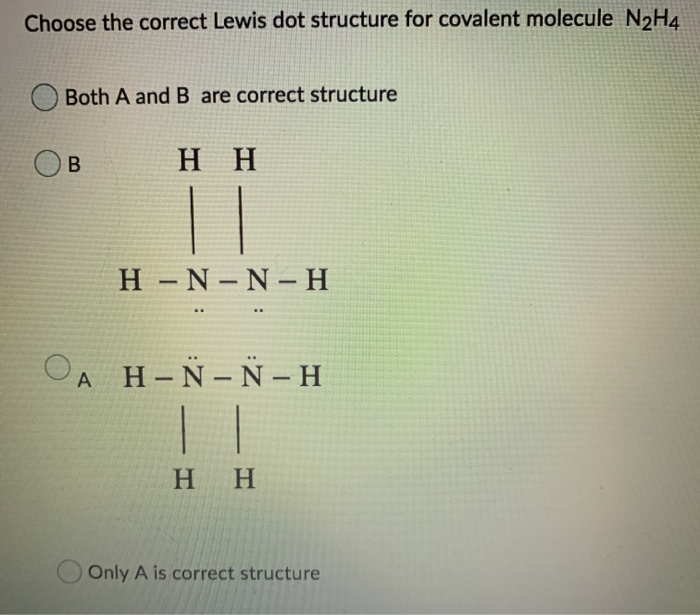
Choose The Correct Lewis Dot Structure For Covalent Chegg Com

Draw The Lewis Structure Of N2h4 And Determine The Hybridization Orbital For Each Element Study Com
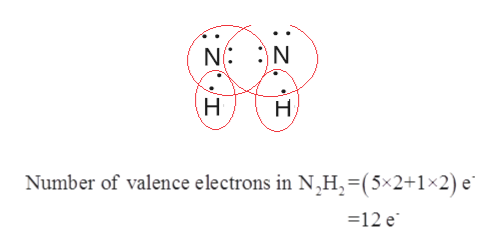
Answered Draw All The Lewis Structures For N2h2 Bartleby

What Is The Lewis Structure For N2h4 Study Com
Why Does N N Dimethylhydrazine Have Hydrazine When It Has N2h2 Not N2h4 Hydrazine Why Isn T It Called Dimethyldiimide Quora
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
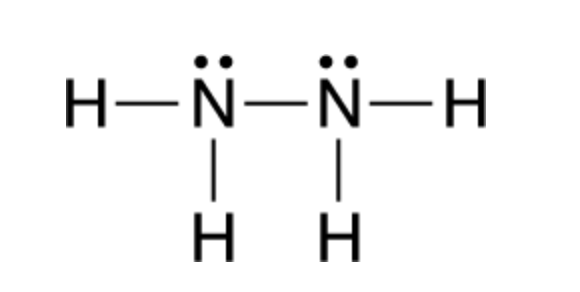
Answer The Following Questions About N2 And N2h4 Chegg Com

Draw The Lewis Structure For N2h4 Clutch Prep