Lewis Electron Structure C2h4
It has 1 valence electron. Lewis Structure Examples.
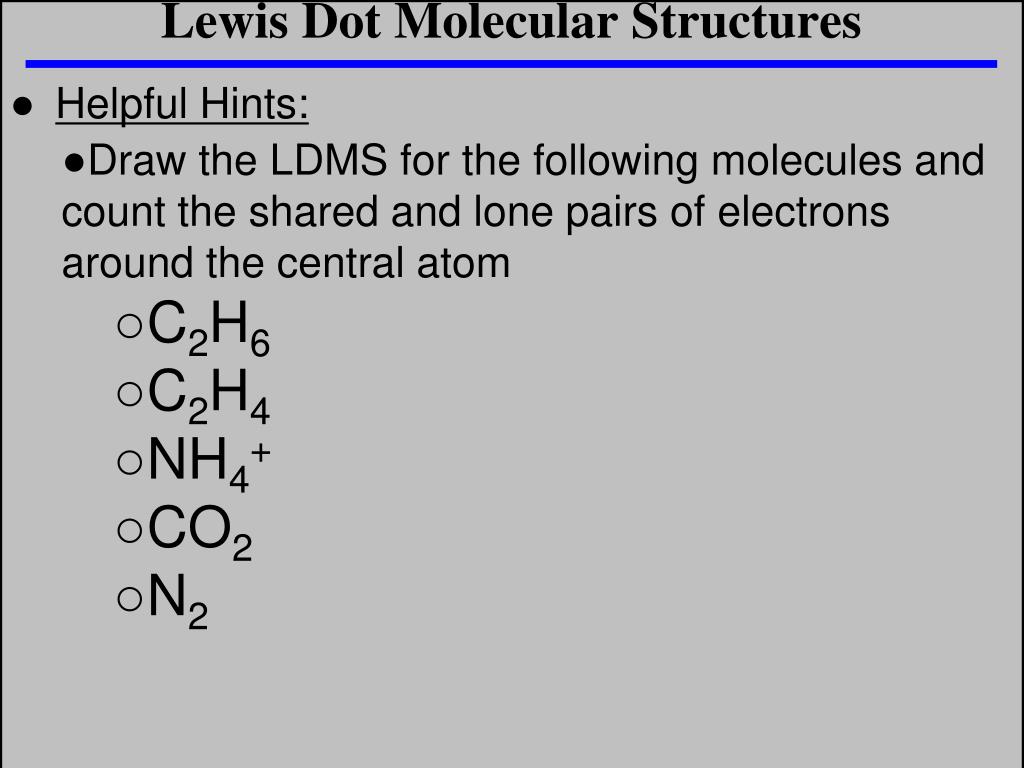
Ppt Lewis Dot Molecular Structures Powerpoint Presentation Free Download Id 4787586
What are the two main components of hexanes.
Lewis electron structure c2h4. If we come way over here to Hydrogen its in group 1. There are two triangles overlapping each other as we can see in. The electron dot structure is drawn using Lewis-dot structure.
Electron Dot Structure for ethane C2H4. It means four CH bond has 8 shared pairs of electrons and CC bond has 4 shared pairs of electrons. Such as polarity and bond length we will draw the lewis or electron dot structure.
Oxygen contains 6 valence electrons which form 2 lone pairs. What is the chemical formula for methane. What is the electron dot formula of C2H4.
H Group 1A 1 valence electron. Alternatively a dot method can be used to draw the lewis structure. C2H4 Lewis Structure Ethene.
Draw the Lewis structure for ethylene C 2 H 4. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. This means that the carbon atoms share 4 electrons.
Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. Draw the Lewis structure for ethylene C2H4. The Lewis formalism used for the H2 molecule is HH or HH.
How many electrons are shared between the carbon atoms in. C bonding preference is 4. 81 445 ratings Problem Details.
Calculate the total valence electrons in the molecule. Sothe total number of CH2CH2 valence electrons is twelve 8 412 These 12 valence electrons have two tasks at the the same timeThey connect all the atoms and satisfy the duet rule of hygrogens and octet rule of two carbon atoms. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them.
It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The key to understanding how to distribute the valence electrons is. As per the Ethene Lewis dot structure Four CH sigma bonds are present and one CC double bond1 sigma 1 pie bond.
By signing up youll get thousands of. What is the Lewis structure of H. The central atom of this molecule is carbon.
Carbon is in group 4 sometimes written 14 so it has 4 valence electrons. Therefore there cannot be more than one stable resonance structure for C 2 H 4. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. For C 2 H 4 you have a total of 12 total valence electrons. C2H4 Lewis Structure Hydrogen is the least electronegative element here.
2C 4 2 8. To do that we always count our valence electrons up first. Since it is bonded to only one carbon atom it must form a double bond.
Is it polar or nonpolar. Neutral Compounds Concept Videos. It is a chemical formula for Ethylene or Ethene.
How many shared pairs of electrons are in the lewis dot structure of C2H4. However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
To draw the c2h4 lewis. Draw the electron-dot structure for C2H4. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells.
In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Electron dot structure of C2H4 H2CCH2. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
The former known as a Lewis dot diagram indicates a pair of shared electrons between the atomic symbols while the latter known as a Lewis structure uses a dash to indicate the pair of shared electrons that form a covalent bond. Lewis Structure of CO 2. Use information from step 4 and 5 to draw the lewis structure.
Lets take a look. Lewis dot structure of C 2 H 4. Learn this topic by watching Lewis Dot Structures.
The Lewis electron dot structures of a few molecules are illustrated in this subsection. When there are no polar bonds in a molecule there is no permanent charge difference between one part of the molecule and another and a. Hence total shared pairs of electrons in the Lewis dot structure of C2H4 is 12.
What is the shape of C2H4. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell.
Its C2H4 and we want to write the dot structures for ethene. It consists of two carbon molecules and 4 hydrogen molecules. What is its molecular shape.
2 1 5 Drawing Lewis Structures Chemistry Libretexts

8 1 Alkene Structure Chemistry Libretexts

H2cch2 Lewis Structure Valence Electrons Formal Charge

H2cch2 Lewis Structure Valence Electrons Formal Charge
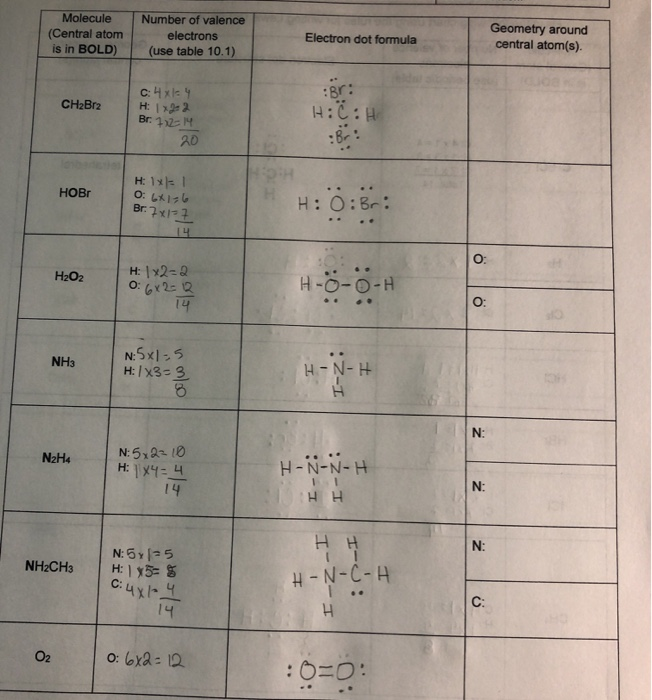
Fill In The Following Chart Using The Rules For Chegg Com

Section 12 3 Lewis Structures 1 To Predict The Electron Arrangement In Molecules 2 To Learn To Write Simple Lewis Structures 3 To Learn To Write Lewis Ppt Download

H2cch2 Lewis Structure Valence Electrons Formal Charge

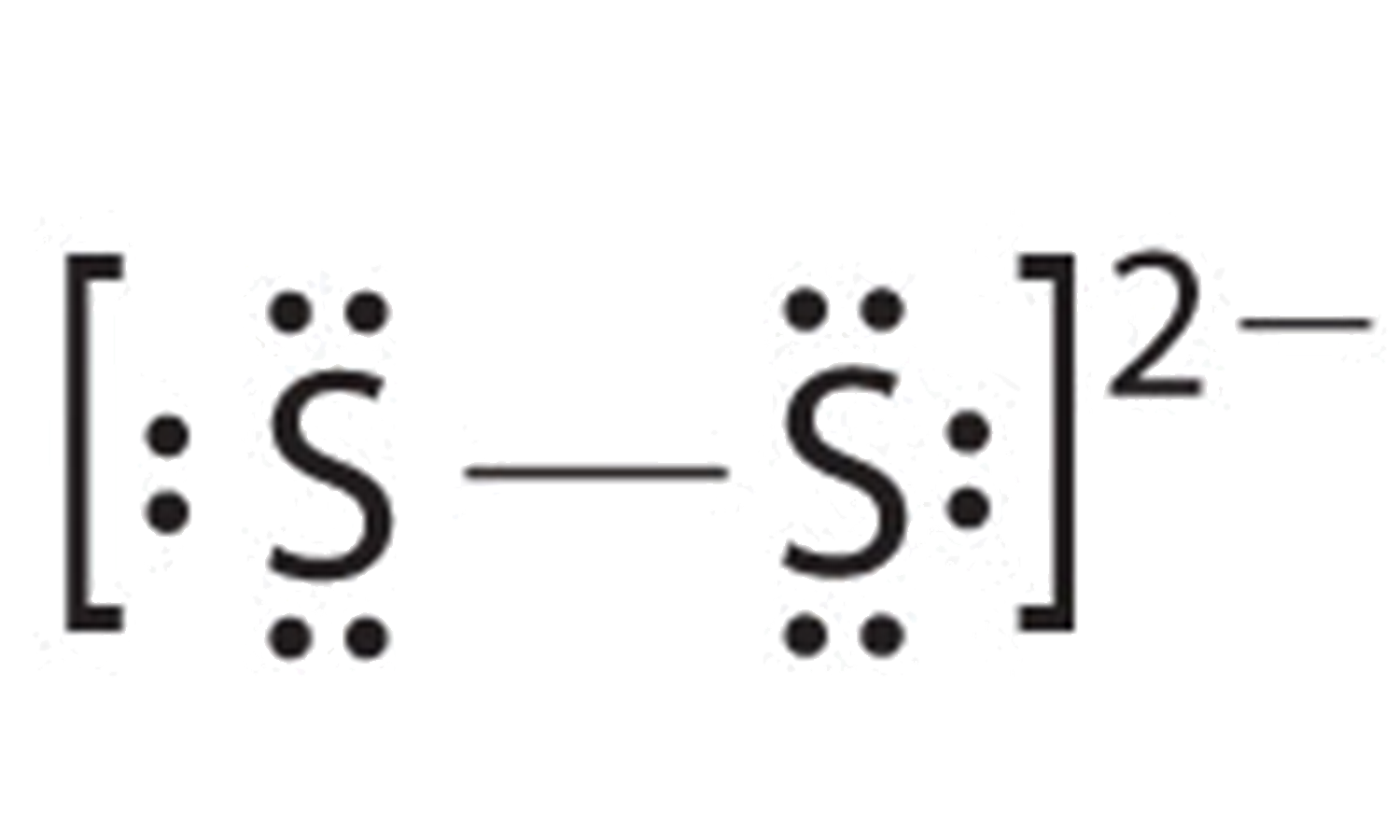
Clo4 Lewis Structure Perchlorate Ion Youtube
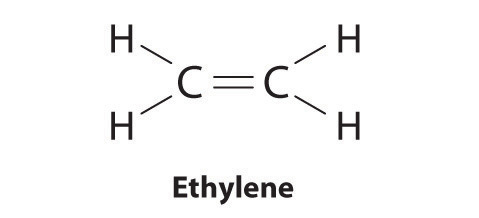
2 1 5 Drawing Lewis Structures Chemistry Libretexts
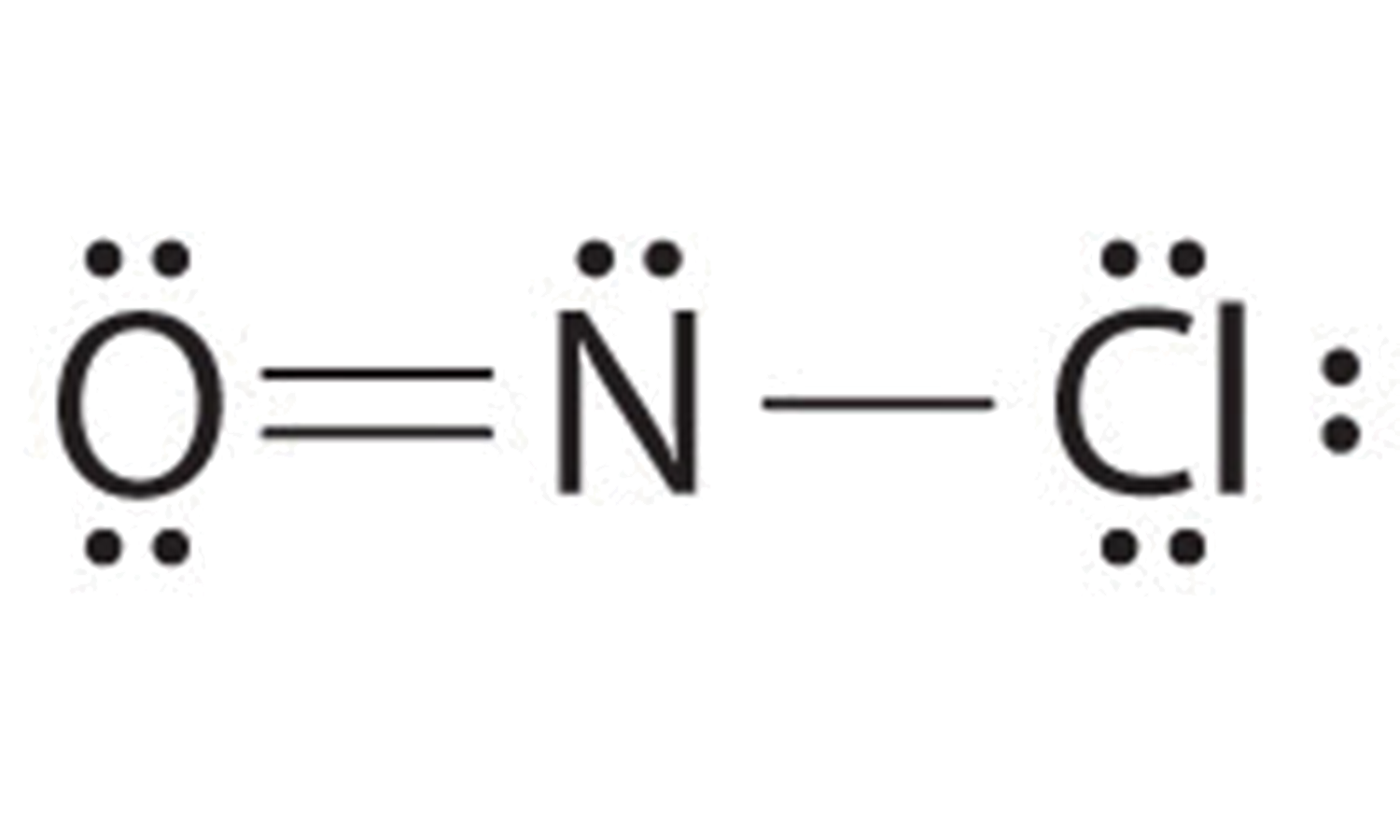
2 1 5 Drawing Lewis Structures Chemistry Libretexts

Vsepr For 4 Electron Clouds Video Vsepr Khan Academy
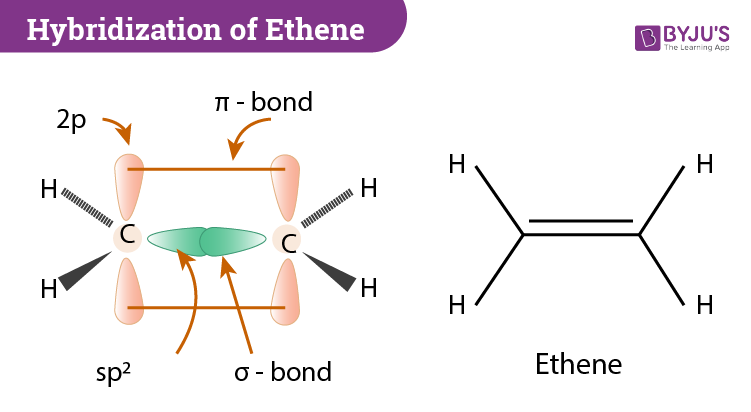
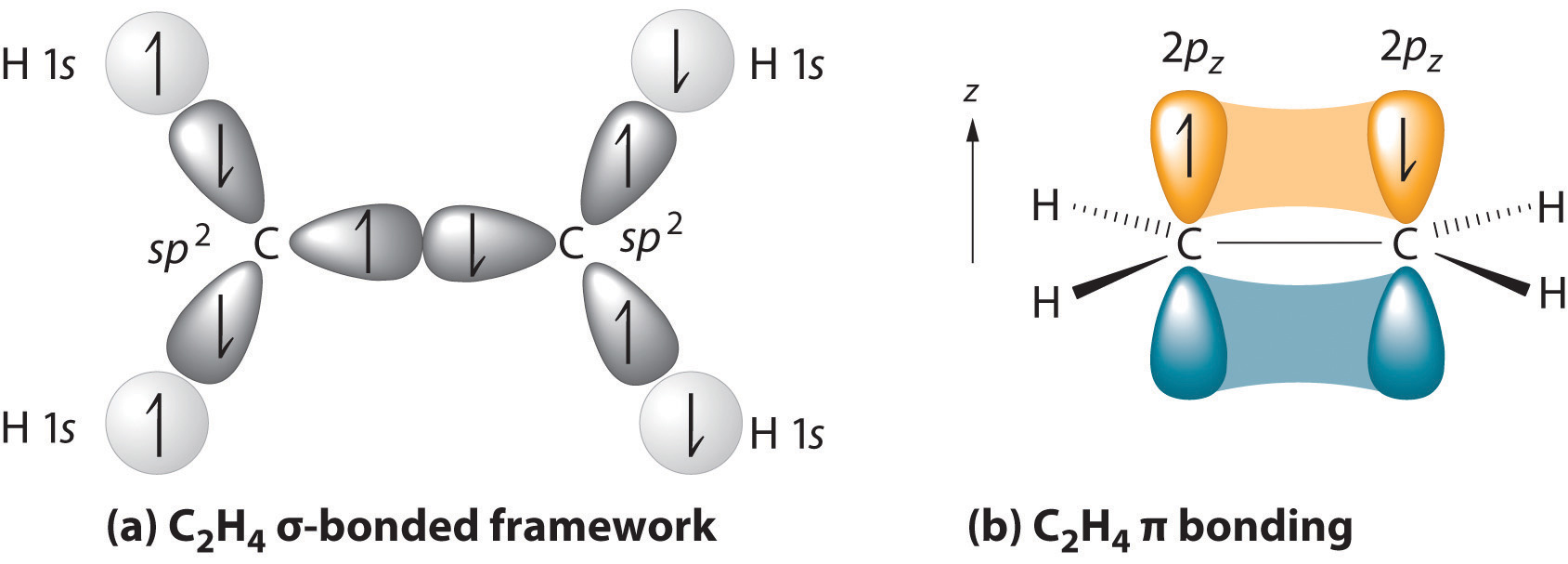
Hybridization Of C2h4 Ethene Hybridization Of Carbon In C2h4
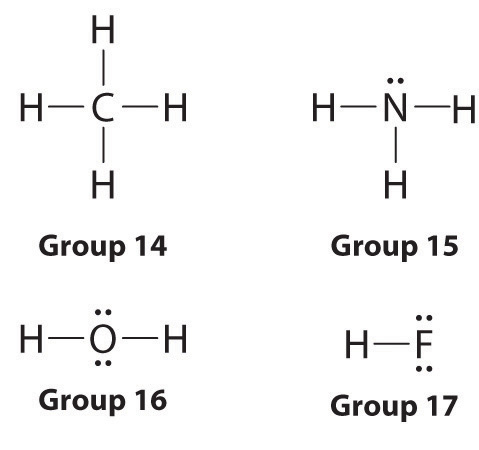
2 1 5 Drawing Lewis Structures Chemistry Libretexts

H2cch2 Lewis Structure Valence Electrons Formal Charge
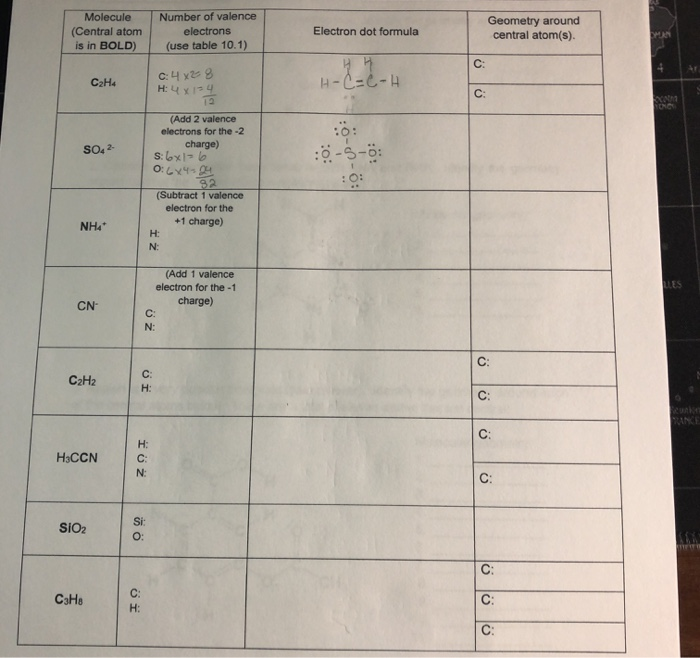
Fill In The Following Chart Using The Rules For Chegg Com
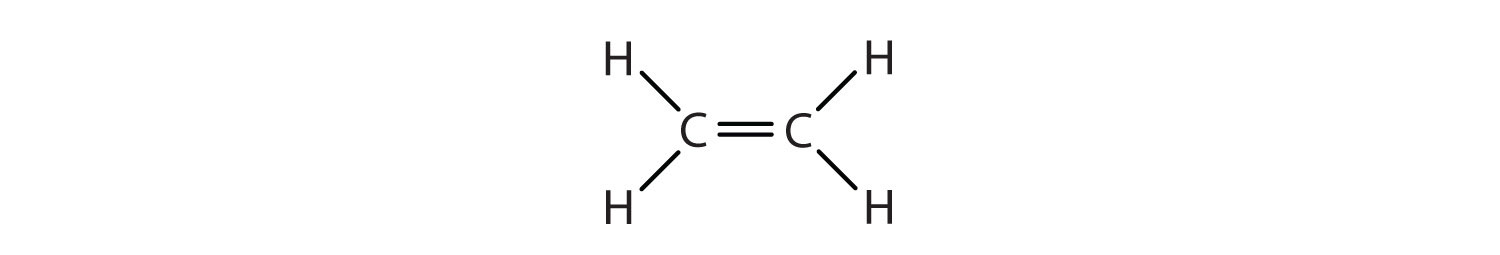
8 1 Alkene Structure Chemistry Libretexts
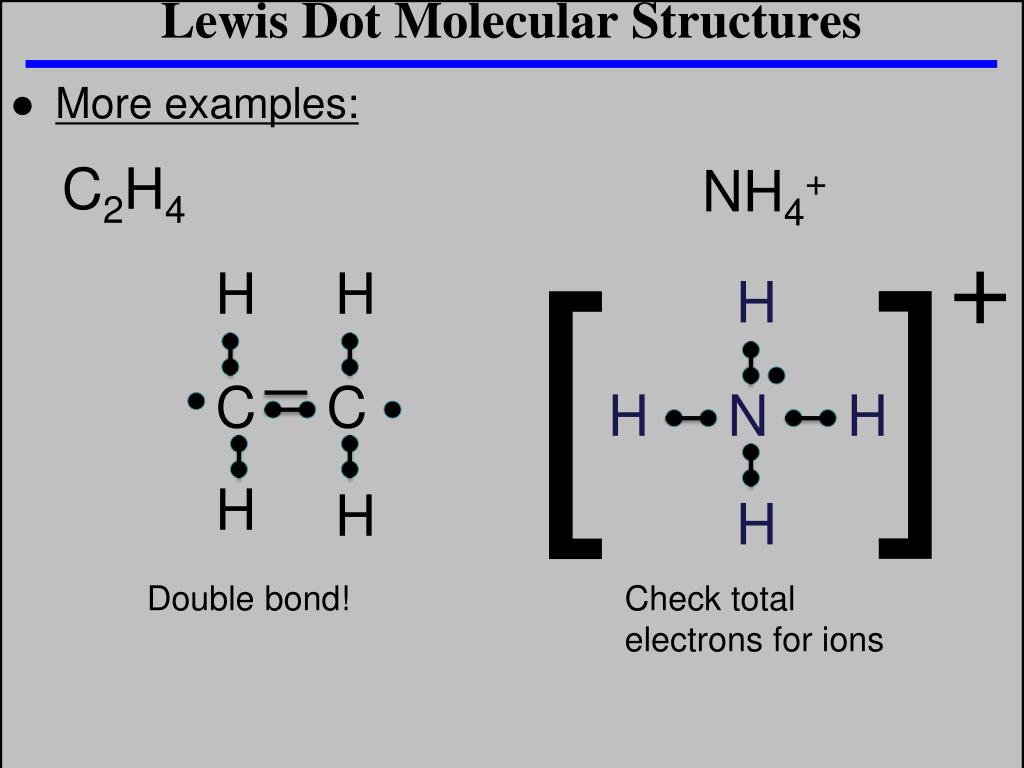
Ppt Lewis Dot Molecular Structures Powerpoint Presentation Free Download Id 4787586

8 1 Alkene Structure Chemistry Libretexts
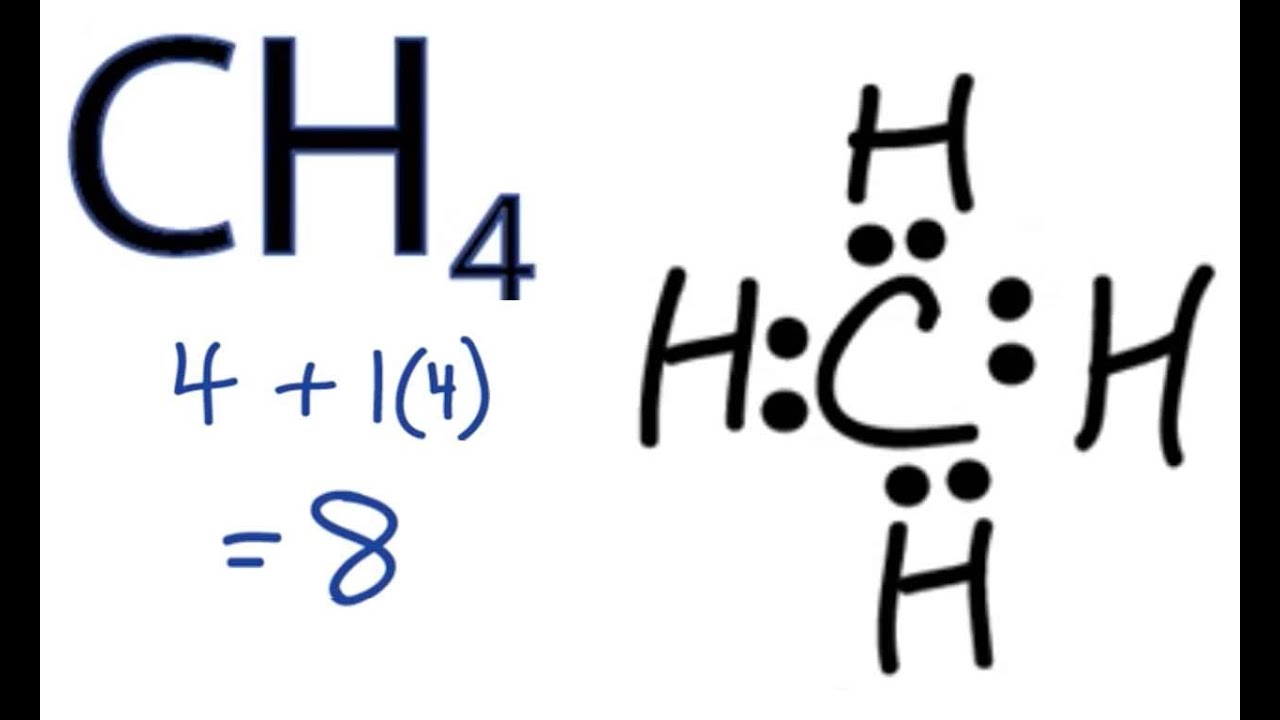
Lewis Structure For Ch4 Methane