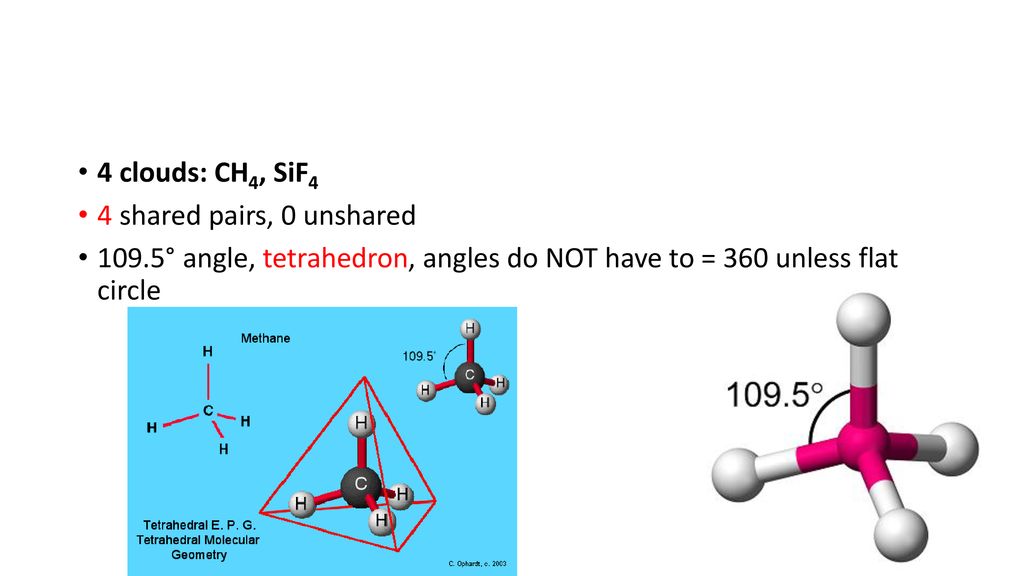
Molecular Geometry For Sif4
Hence SF4 has a trigonal bipyramidal molecular geometry. QUIZ4C-CH10-MG - Prince Georges Community College.
Today S Do Now Write The Lewis Dot Diagram For The Ionic Bond Between Sodium And Sulfur Write The Lewis Structure For The H2s Molecule Do Resonance Ppt Video Online Download
Silicon Tetrafluoride 7783-61-1.

Molecular geometry for sif4. Predict the molecular geometry of the compound SiF4 using VSEPR. In SiF4 four Si-F bonds are formed during the molecular formation in such a way that Si atom is in a central position surrounded by four F atoms with a bond angle of 1095 which makes the SiF4 molecule symmetrical. Electron And Molecular Geometry Revision 1 Admirable Electron and molecular geometry illustration Electron And Molecular Geometry Hqdefault Delectable 1 Part.
Use the electron dot structure Lewis structure and the molecular shape table to determine the molecular shape molecular geometry. And as there is no movement in this molecule there are no poles formed in this molecule. Its boiling point is only 4 C above its melting point.
These charges or this diaper moments cancel each other out. It is a tetrahedral molecule. SiF4 Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar.
7 Zeilen SiF4 Lewis Structure Molecular Geometry Hybridization and Polarity. Sif4 lewis structure how to draw the lewis dot structures of covalent compound yeah. The electron geometry for.
This colorless compound is notable for having a narrow liquid range. And hence SIF four is a nonpolar molecule. The electron geometry for.
SIF4 is a covalent. The geometrical symmetry of Sif4 plays vital role in making it a non-polar molecule. If playback doesnt begin shortly try restarting your device.
Computed by Cactvs 34611 PubChem release 20190618 Rotatable Bond Count. Computed by Cactvs 34611 PubChem release 20190618 Exact Mass. Lone pair electrons of central atom.
But as it ah it is a symmetrical molecule. So there is no dipole moment observed. SBr2 Molecular Geometry Shape and Bond Angles.
Hence Si atom is sp3 hybridized in this compound and SiF4 gets tetrahedral molecular geometry. What is the Lewis structure for SiF4. Sif4 Lewis Structure Related Keywords Amp Suggestions For Sicl4 sif4 lewis structure how to draw the lewis dot structures of covalent compound yeah chemistry.
Silicon tetrafluoride or tetrafluorosilane is the chemical compound with the formula SiF4. It causes charges produced on the molecule to cancel each others out and hence net becomes zero. Sif4 molecule has a tetrahedral molecular geometry.
SiF4 molecule has a tetrahedral molecular geometry. Draw the Lewis structure of silicon. Pin - ppt video online download 10 For.
Ch3br Electron Geometry - Drone Fest. SF4 Lewis Structure Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. By signing up youll get thousands of step-by-step solutions to your.
It was first synthesized by John Davy in 1812. Molecular Shape of SiF 4 Answer. In sif4 four si f bonds are formed during the molecular formation in such a way that si atom is in a central position surrounded by four f atoms with a bond angle of 1095 which makes the sif4 molecule symmetrical.
Therefore the total dipole movement of molecule becomes zero and dont show polar characteristics. In SiF4 the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. I hope this video helps you to understand that and for more such videos on lowest structure molecule or geometry polarity of the molecules.
Computed by Cactvs 34611 PubChem release 20190618 Hydrogen Bond Acceptor Count. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count. So the steric number of Si is 4.
An explanation of the molecular geometry for the SiF4 ion Silicon tetrafluoride including a description of the SiF4 bond angles. Some Useful Properties of SiF4. Draw a Lewis structure for a SiF4.
Lone pair electrons of central atom. The molecular shapes of SF4 SiF4 and ICl4- are. And as these diaper moments are cancelled out the moment of this molecule becomes zero.
Sif4 molecular geometry. Molecular Shape of H 2 CO Answer. SiF4 is not polar as the fluorines negative dipoles cancel each other out as the are all pulling away form the centre equally the centre being silicon which has a lower electronegativity than fluorine.
Determine the electron geometry eg and molecular geometry. Atoms bonded to central atom. 2 pairs 4 electrons total Molecular Shape of SH 2 Answer.
A step-by-step explanation of how to draw the SiH4 Lewis Dot Structure Silicon TetrafluorideFor the SiH4 structure use the periodic table to find the tota. Atoms bonded to central atom.

Silicon Tetrafluoride Sif4 Lewis Dot Structure Youtube
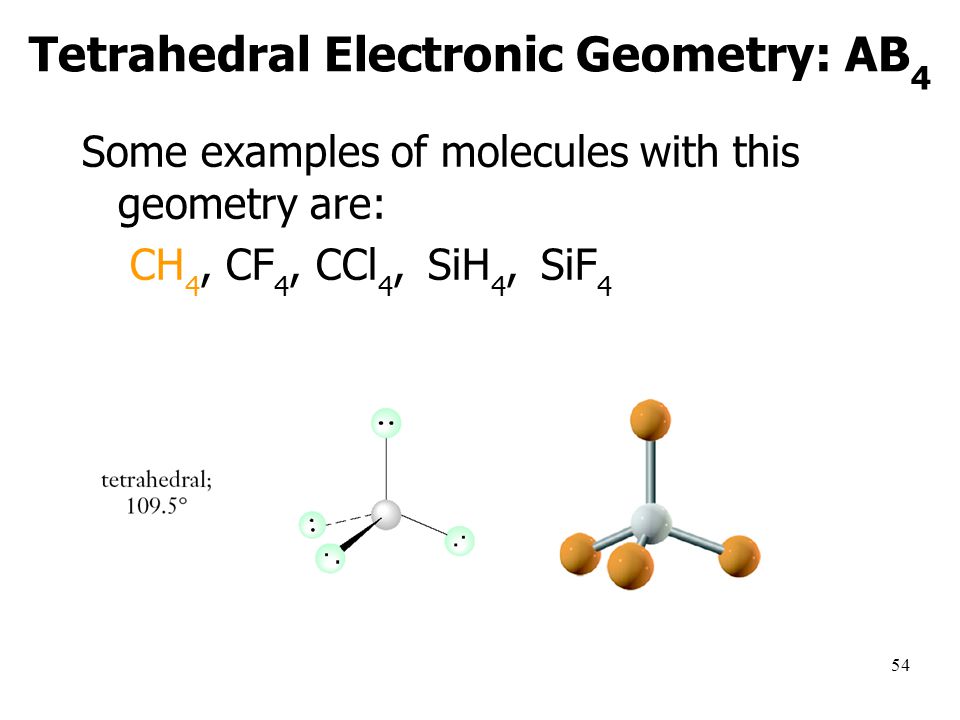
Bonding And Molecular Structure Ppt Download
What Is The Molecular Geometry Of Sif4 Quora
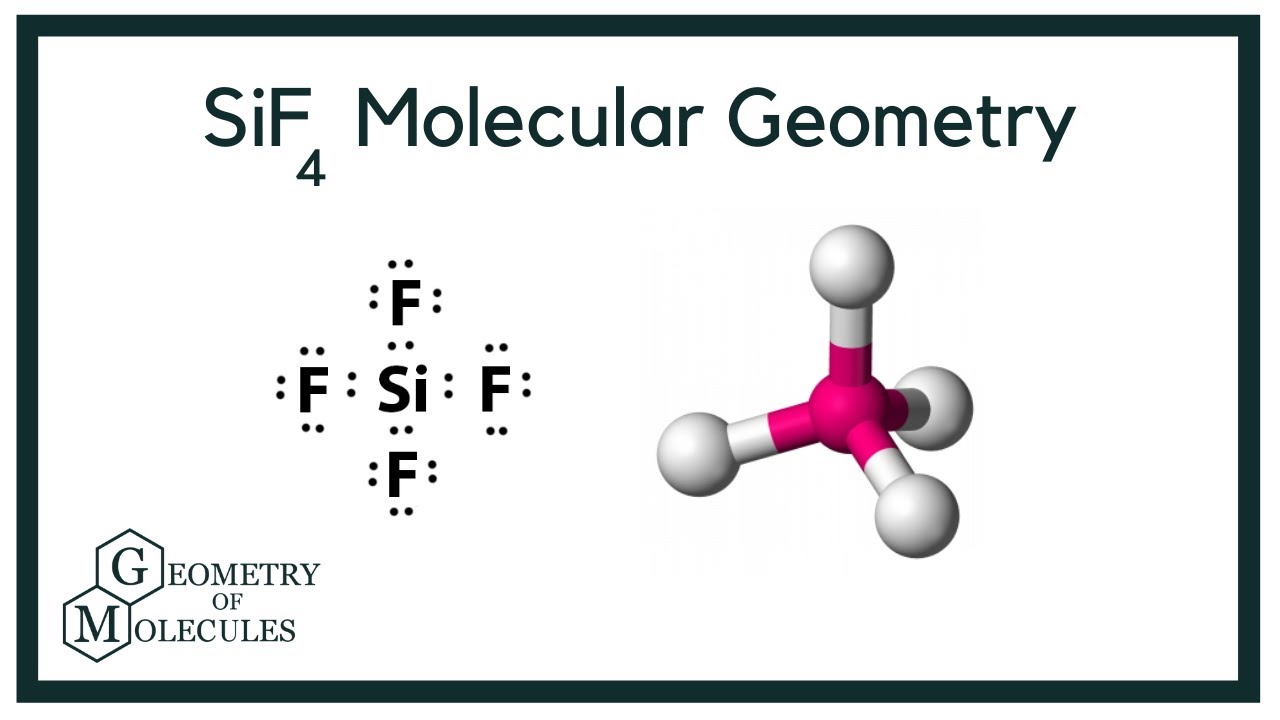
Sif4 Molecular Geometry Bond Angles Electron Geometry Silicon Tetrafluoride Youtube

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
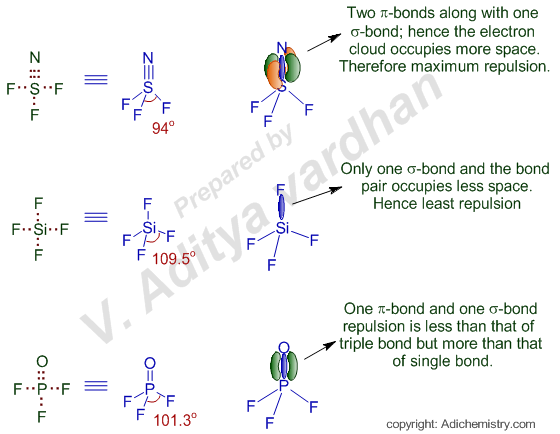
Vsepr Theory Bond Angles Nsf3 Sif4 Pof3

For Each Of The Molecules Below Determine The Chegg Com

What Is The Molecular Geometry Of Sf4 Quora
Chem Molecular Shape Molecular Geometry Scientific Tutor

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Silicon Tetrafluoride 7783 61 1

Sif4 Molecular Geometry Bond Angles Electron Geometry Youtube

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Sif4 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
7 10 Notes Shapes For Covalent Structures Ppt Download

